Histone H3 phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK in rat hepatocytes exposed to ethanol and acetaldehyde
- PMID: 17643407
- PMCID: PMC2723821
- DOI: 10.1016/j.ejphar.2007.06.049
Histone H3 phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK in rat hepatocytes exposed to ethanol and acetaldehyde
Abstract
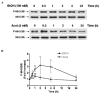
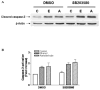
Ethanol modulates mitogen-activated protein kinases (MAPKs). We have now investigated the influence of ethanol and its metabolite, acetaldehyde on histone H3 phosphorylation to ascertain downstream targets of MAPKs. In primary culture of rat hepatocytes, ethanol and acetaldehyde increased phosphorylation of nuclear histone H3 at serine 10 and serine 28. Specific inhibitors of p38 MAPK, SB203580, PD169316 and SB202190 blocked this phosphorylation. The inactive analogue, SB202474 had no effect. In contrast, c-Jun N-terminal kinase (JNK) inhibitor, SP600125 or MAP/ERK kinase (MEK) 1/2 inhibitor, PD98059 had no effect on the histone H3 phosphorylation. The p38 MAPK activation correlated with upstream activation of MAPK kinase (MKK) 3/6 but was independent of protein synthesis. In the nuclear fraction, the phosphorylation of p38 MAPK and its protein level increased with peak activation at 24 h by ethanol and at 30 min by acetaldehyde. These responses were ethanol and acetaldehyde dose dependent. Surprisingly, the phosphorylation of p38 MAPK was undetectable in the cytosolic fraction suggesting a subcellular selectivity of p38 MAPK signaling. The phosphorylation of JNK and p42/44 MAPK and their protein levels also increased in the nuclear fraction. Although ethanol caused translocation of all three major MAPKs (p42/44 MAPK, JNK, p38 MAPK) into the nucleus, histone H3 phosphorylation at serine 10 and serine 28 was mediated by p38 MAPK. This histone H3 phosphorylation had no influence on ethanol and acetaldehyde induced apoptosis. These studies demonstrate for the first time that ethanol and acetaldehyde stimulated phosphorylation of histone H3 at serine 10 and serine 28 are downstream nuclear response mediated by p38 MAPK in hepatocytes.
Figures








Similar articles
-
Pro- and anti-apoptotic roles of c-Jun N-terminal kinase (JNK) in ethanol and acetaldehyde exposed rat hepatocytes.Eur J Pharmacol. 2005 Jan 31;508(1-3):31-45. doi: 10.1016/j.ejphar.2004.12.006. Epub 2005 Jan 13. Eur J Pharmacol. 2005. PMID: 15680252
-
Involvement of histone H3 phosphorylation through p38 MAPK pathway activation in casticin-induced cytocidal effects against the human promyelocytic cell line HL-60.Int J Oncol. 2013 Dec;43(6):2046-56. doi: 10.3892/ijo.2013.2106. Epub 2013 Sep 20. Int J Oncol. 2013. PMID: 24064676
-
Pancreatic stellate cell activation by ethanol and acetaldehyde: is it mediated by the mitogen-activated protein kinase signaling pathway?Pancreas. 2003 Aug;27(2):150-60. doi: 10.1097/00006676-200308000-00008. Pancreas. 2003. PMID: 12883264
-
MAP kinase signaling in diverse effects of ethanol.Life Sci. 2004 Mar 26;74(19):2339-64. doi: 10.1016/j.lfs.2003.11.001. Life Sci. 2004. PMID: 15027449 Review.
-
Is p38 MAPK Associated to Drugs of Abuse-Induced Abnormal Behaviors?Int J Mol Sci. 2020 Jul 8;21(14):4833. doi: 10.3390/ijms21144833. Int J Mol Sci. 2020. PMID: 32650599 Free PMC article. Review.
Cited by
-
Genetic and epigenetic insights into fetal alcohol spectrum disorders.Genome Med. 2010 Apr 28;2(4):27. doi: 10.1186/gm148. Genome Med. 2010. PMID: 20423530 Free PMC article.
-
Quantifying Competition among Mitochondrial Protein Acylation Events Induced by Ethanol Metabolism.J Proteome Res. 2019 Apr 5;18(4):1513-1531. doi: 10.1021/acs.jproteome.8b00800. Epub 2019 Jan 31. J Proteome Res. 2019. PMID: 30644754 Free PMC article.
-
Silver Nanoparticle-Induced Phosphorylation of Histone H3 at Serine 10 Involves MAPK Pathways.Biomolecules. 2019 Feb 22;9(2):78. doi: 10.3390/biom9020078. Biomolecules. 2019. PMID: 30813344 Free PMC article.
-
Epigenetic regulation in alcoholic liver disease.World J Gastroenterol. 2011 May 28;17(20):2456-64. doi: 10.3748/wjg.v17.i20.2456. World J Gastroenterol. 2011. PMID: 21633650 Free PMC article. Review.
-
Mechanism and Therapeutic Opportunities of Histone Modifications in Chronic Liver Disease.Front Pharmacol. 2021 Nov 23;12:784591. doi: 10.3389/fphar.2021.784591. eCollection 2021. Front Pharmacol. 2021. PMID: 34887768 Free PMC article. Review.
References
-
- Ahn NG, Seger R, Bratlien RL, Diltz CD, Tonks NK, Krebs EG. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991;266:4220–4227. - PubMed
-
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. - PubMed
-
- Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JM, Davie JR. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed Mouse Fibroblasts. J Biol Chem. 1999;274:24914–24920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

