Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation
- PMID: 17643448
- PMCID: PMC2045504
- DOI: 10.1016/j.yjmcc.2007.05.022
Ca2+/calmodulin kinase II increases ryanodine binding and Ca2+-induced sarcoplasmic reticulum Ca2+ release kinetics during beta-adrenergic stimulation
Abstract
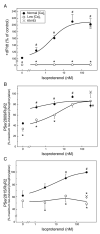
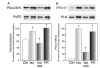
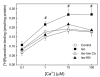
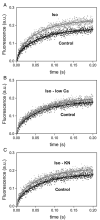
We aimed to define the relative contribution of both PKA and Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) cascades to the phosphorylation of RyR2 and the activity of the channel during beta-adrenergic receptor (betaAR) stimulation. Rat hearts were perfused with increasing concentrations of the beta-agonist isoproterenol in the absence and the presence of CaMKII inhibition. CaMKII was inhibited either by preventing the Ca(2+) influx to the cell by low [Ca](o) plus nifedipine or by the specific inhibitor KN-93. We immunodetected RyR2 phosphorylated at Ser2809 (PKA and putative CaMKII site) and at Ser2815 (CaMKII site) and measured [(3)H]-ryanodine binding and fast Ca(2+) release kinetics in sarcoplasmic reticulum (SR) vesicles. SR vesicles were isolated in conditions that preserved the phosphorylation levels achieved in the intact heart and were actively and equally loaded with Ca(2+). Our results demonstrated that Ser2809 and Ser2815 of RyR2 were dose-dependently phosphorylated under betaAR stimulation by PKA and CaMKII, respectively. The isoproterenol-induced increase in the phosphorylation of Ser2815 site was prevented by the PKA inhibitor H-89 and mimicked by forskolin. CaMKII-dependent phosphorylation of RyR2 (but not PKA-dependent phosphorylation) was responsible for the beta-induced increase in the channel activity as indicated by the enhancement of the [(3)H]-ryanodine binding and the velocity of fast SR Ca(2+) release. The present results show for the first time a dose-dependent increase in the phosphorylation of Ser2815 of RyR2 through the PKA-dependent activation of CaMKII and a predominant role of CaMKII-dependent phosphorylation of RyR2, over that of PKA-dependent phosphorylation, on SR-Ca(2+) release during betaAR stimulation.
Figures

References
-
- Mundiña-Weilenmann C, Vittone L, Ortale M, de Cingolani GC, Mattiazzi A. Immunodetection of phosphorylation sites gives new insights into the mechanisms underlying phospholamban phosphorylation in the intact heart. J Biol Chem. 1996;271:33561–7. - PubMed
-
- Said M, Mundiña-Weilenmann C, Vittone L, Mattiazzi A. The relative relevance of phosphorylation of the Thr17 residue of phospholamban is different at different levels of β-adrenergic stimulation. Pflugers Arch. 2002;444:801–9. - PubMed
-
- Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, et al. Sustained β1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004;95:798–806. - PubMed
-
- Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol. 2006;291:H1299–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

