Understanding morphogen gradients: a problem of dispersion and containment
- PMID: 17643982
- PMCID: PMC1993832
- DOI: 10.1016/j.gde.2007.05.010
Understanding morphogen gradients: a problem of dispersion and containment
Abstract
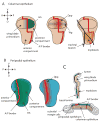
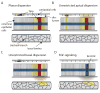
Protein morphogens are instructive signals that regulate growth and patterning of tissues and organs. They form long-range, dynamic gradients by moving from regions of high concentration (producing cells) to regions of low concentration (the adjacent, nonproducing developmental field). Since morphogen activity must be limited to the adjacent target field, we want to understand both how signaling proteins move and how their dispersion is restricted. We consider the variety of settings for long-range morphogen systems in Drosophila. In the early embryo, morphogens appear to disperse by free diffusion, and impermeable membranes physically constrain them. However, at later stages, containment is achieved without physical barriers. We argue that in the absence of constraining barriers, gradient-generating dispersion of morphogens cannot be achieved by passive diffusion and that other mechanisms for distribution must be considered.
Figures



References
-
- Martinez Arias A. Wnts as morphogens? The view from the wing of Drosophila. Nat Rev Mol Cell Biol. 2003;4:321–325. - PubMed
-
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. - PubMed
-
- Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. - PubMed
-
- Zhu AJ, Scott MP. Incredible journey: how do developmental signals travel through tissue? Genes Dev. 2004;18:2985–2997. - PubMed
-
- Eaton S. Release and trafficking of lipid-linked morphogens. Curr Opin Genet Dev. 2006;16:17–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

