Effect of neural precursor proliferation level on neurogenesis in rat brain during aging and after focal ischemia
- PMID: 17644223
- PMCID: PMC2634816
- DOI: 10.1016/j.neurobiolaging.2007.06.004
Effect of neural precursor proliferation level on neurogenesis in rat brain during aging and after focal ischemia
Abstract
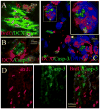
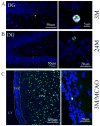
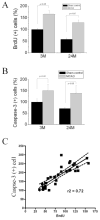
The observed age-related decline in neurogenesis may result from reduced proliferation or increased death rate of neuronal precursor cells (NPCs). We found that caspase-3, but not caspase-6, -7, or -9, was activated in NPCs in neurogenic regions of young, young-adult, middle-aged and aged rat brains. The number of capase-3-immunoreactive cells was highest in young and lowest in aged rats. Surprisingly, intraventricular administration of a caspase-3 inhibitor failed to restore the number of BrdU-positive cells in the aged dentate gyrus, suggesting that the age-related decline in neurogenesis may be attributable primarily to reduced proliferation. Additionally, we also found that NPCs in the subventricular zone of young-adult and aged rat brain were increased after focal cerebral ischemia, suggesting that the increase in neurogenesis induced by ischemia may result from an increase in the rate of NPC proliferation, but not from a decrease in NPC death. Thus, our results suggest that age-related and injury-induced changes in the rate of neurogenesis are controlled at the level of NPC proliferation. Furthermore, our results may imply that the mechanisms that maintain a stable population of NPCs in the normal adult and in the ischemic brain, which account for the observed age-dependent reduction or injury-induced increases in neurogenesis, impinge on the regulation of cell division at the NPC level.
Conflict of interest statement
Disclosure Statement
There are no any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence (bias) our work. All animal experiments were carried out in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the IACUC. Efforts were made to minimize animal suffering and to reduce the number of animals used.
Figures



Similar articles
-
Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia.Aging Cell. 2013 Dec;12(6):978-87. doi: 10.1111/acel.12134. Epub 2013 Aug 6. Aging Cell. 2013. PMID: 23834718 Free PMC article.
-
Temporal profile of neurogenesis in the subventricular zone, dentate gyrus and cerebral cortex following transient focal cerebral ischemia.Neurol Res. 2009 Nov;31(9):969-76. doi: 10.1179/174313209X383312. Epub 2009 Jan 9. Neurol Res. 2009. PMID: 19138475
-
Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats.J Cereb Blood Flow Metab. 2002 Mar;22(3):299-307. doi: 10.1097/00004647-200203000-00007. J Cereb Blood Flow Metab. 2002. PMID: 11891435
-
Forebrain neurogenesis after focal Ischemic and traumatic brain injury.Neurobiol Dis. 2010 Feb;37(2):267-74. doi: 10.1016/j.nbd.2009.11.002. Epub 2009 Nov 10. Neurobiol Dis. 2010. PMID: 19909815 Free PMC article. Review.
-
Brain ischemia, neurogenesis, and neurotrophic receptor expression in primates.Arch Ital Biol. 2011 Jun;149(2):225-31. doi: 10.4449/aib.v149i2.1368. Arch Ital Biol. 2011. PMID: 21701994 Review.
Cited by
-
Current perspectives on the link between neuroinflammation and neurogenesis.Metab Brain Dis. 2015 Apr;30(2):355-65. doi: 10.1007/s11011-014-9523-6. Epub 2014 Mar 13. Metab Brain Dis. 2015. PMID: 24623361 Review.
-
Pharmacological approaches to intervention in hypomyelinating and demyelinating white matter pathology.Neuropharmacology. 2016 Nov;110(Pt B):605-625. doi: 10.1016/j.neuropharm.2015.06.008. Epub 2015 Jun 24. Neuropharmacology. 2016. PMID: 26116759 Free PMC article. Review.
-
AMPK Signaling Regulates the Age-Related Decline of Hippocampal Neurogenesis.Aging Dis. 2019 Oct 1;10(5):1058-1074. doi: 10.14336/AD.2019.0102. eCollection 2019 Oct. Aging Dis. 2019. PMID: 31595203 Free PMC article.
-
Adult Neurogenesis in the Subventricular Zone and Its Regulation After Ischemic Stroke: Implications for Therapeutic Approaches.Transl Stroke Res. 2020 Feb;11(1):60-79. doi: 10.1007/s12975-019-00717-8. Epub 2019 Jul 15. Transl Stroke Res. 2020. PMID: 31309427 Review.
-
Developmental profiling of postnatal dentate gyrus progenitors provides evidence for dynamic cell-autonomous regulation.Hippocampus. 2011 Jan;21(1):33-47. doi: 10.1002/hipo.20719. Hippocampus. 2011. PMID: 20014381 Free PMC article.
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–70. - PubMed
-
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291(1):17–20. - PubMed
-
- Biebl M, Winner B, Winkler J. Caspase inhibition decreases cell death in regions of adult neurogenesis. Neuroreport. 2005;16(11):1147–50. - PubMed
-
- Bingham B, Liu D, Wood A, Cho S. Ischemia-stimulated neurogenesis is regulated by proliferation, migration, differentiation and caspase activation of hippocampal precursor cells. Brain Res. 2005;1058(1–2):167–77. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

