Assessment of rituximab's immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results
- PMID: 17644541
- PMCID: PMC2754142
- DOI: 10.1136/ard.2007.074229
Assessment of rituximab's immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results
Abstract
Objective: Treatment with the anti-CD20 monoclonal antibody (mAb) rituximab is effective in rheumatoid arthritis (RA). Marked depletion of circulating B cells, seen in almost all patients, does not correlate with efficacy. The potential synovial immunomodulatory effects of rituximab have not been fully defined.
Methods: The ARISE trial is an open label, serial synovial biopsy (pre-treatment and 8 weeks) study of rituximab, given 1 g intravenously on days 0 and 14 without peri-infusional steroids, in active RA patients on concomitant methotrexate (MTX). Synovial tissue was analysed by immunohistochemistry with digital image analysis and gene expression by real-time PCR.
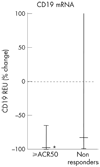
Results: The mean (SD) baseline DAS28 score was 6.5 (0.4), and mean MTX dose 17.3 mg/week. Of 13 patients, 11 had failed prior tumour necrosis factor (TNF) inhibitor therapy. With treatment, all patients experienced near complete depletion of circulating B cell numbers. During the 6 months after treatment, 7/13 patients achieved an American College of Rheumatology (ACR) 20% improvement (ACR20) response, 3/13 an ACR50 response and 2/13 an ACR70 response. There was a significant decrease in synovial B cells after treatment, but only a small trend towards greater reduction among clinical responders. Among the three patients with ACR50 responses there was a significant decrease in synovial immunoglobulin synthesis.
Conclusions: These data suggest that unlike those in circulation, synovial B cells are decreased but are not eliminated by rituximab therapy. Patients with higher levels of response may have more consistent depletion of synovial B cells, and may also have an alteration in synovial B cell function, as indicated by decreases in synovial immunoglobulin synthesis. Thus, effects on synovial B cells may be necessary but not sufficient for inducing clinical efficacy. Other effects, such as on primary lymph organ B cell antigen presentation or cytokine production, may be operative.
Figures







References
-
- Kneitz C, Wilhelm M, Tony HP. Effective B cell depletion with rituximab in the treatment of autoimmune diseases. Immunobiology. 2002;206:519–527. - PubMed
-
- Silverman GJ, Weisman S. Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum. 2003;48:1484–1492. - PubMed
-
- Edwards JCW, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunology. 2006;6:394–503. - PubMed
-
- Silverman GJ. Therapeutic B cell depletion and regeneration in rheumatoid arthritis: emerging patterns and paradigms. Arthritis Rheum. 2006;54:2356–2367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

