Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy?
- PMID: 17644617
- PMCID: PMC2657957
- DOI: 10.2337/dc07-0850
Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy?
Abstract
Objective: We sought to evaluate the prevalence of subclinical neuropathy in intensive and conventional treatment groups at completion of the Diabetes Control and Complications Trial (DCCT).
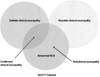
Research design and methods: We assessed neuropathy using nerve conduction results obtained at DCCT completion after stratifying the DCCT cohort to exclude subjects with progressively less severe degrees of diagnosable neuropathy. We began with those who had confirmed clinical neuropathy (the primary DCCT end point) and eventually excluded all subjects with any clinical or electrodiagnostic evidence of neuropathy.
Results: After excluding subjects with confirmed clinical neuropathy at DCCT completion, 8 of 10 nerve conduction measures (including all lower-extremity measures) were significantly improved in the intensive treatment group (O'Brien rank-sum test across all nerve conduction measures, P < 0.0001). Conduction velocity group differences were substantial, and the peroneal conduction velocity averaged 3.1 m/s faster in the intensive compared with the conventional treatment group (45.1 vs. 42.0 m/s, P < 0.0001). Numerous significant differences in median and peroneal motor conduction velocities favoring the intensive treatment group persisted, regardless of the exclusion criteria applied.
Conclusions: Intensive and conventional treatment group subjects without diagnosable neuropathy at DCCT completion had significant differences in electrophysiologic measurements favoring the intensive treatment group. Differences in subsequent incident neuropathy between the original treatment groups may reflect, in part, their levels of subclinical neuropathy at DCCT completion, rather than persistent metabolic effects.
Figures

References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. - PubMed
-
- Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Epidemiology of Diabetes Interventions and Complications (EDIC) design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. - PMC - PubMed
-
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. - PMC - PubMed
-
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

