Spitzenkorper localization and intracellular traffic of green fluorescent protein-labeled CHS-3 and CHS-6 chitin synthases in living hyphae of Neurospora crassa
- PMID: 17644657
- PMCID: PMC2043383
- DOI: 10.1128/EC.00088-07
Spitzenkorper localization and intracellular traffic of green fluorescent protein-labeled CHS-3 and CHS-6 chitin synthases in living hyphae of Neurospora crassa
Abstract
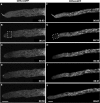
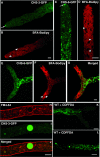
The subcellular location and traffic of two selected chitin synthases (CHS) from Neurospora crassa, CHS-3 and CHS-6, labeled with green fluorescent protein (GFP), were studied by high-resolution confocal laser scanning microscopy. While we found some differences in the overall distribution patterns and appearances of CHS-3-GFP and CHS-6-GFP, most features were similar and were observed consistently. At the hyphal apex, fluorescence congregated into a conspicuous single body corresponding to the location of the Spitzenkörper (Spk). In distal regions (beyond 40 microm from the apex), CHS-GFP revealed a network of large endomembranous compartments that was predominantly comprised of irregular tubular shapes, while some compartments were distinctly spherical. In the distal subapex (20 to 40 microm from the apex), fluorescence was observed in globular bodies that appeared to disintegrate into vesicles as they advanced forward until reaching the proximal subapex (5 to 20 microm from the apex). CHS-GFP was also conspicuously found delineating developing septa. Analysis of fluorescence recovery after photobleaching suggested that the fluorescence of the Spk originated from the advancing population of microvesicles (chitosomes) in the subapex. The inability of brefeldin A to interfere with the traffic of CHS-containing microvesicles and the lack of colocalization of CHS-GFP with the endoplasmic reticulum (ER)-Golgi body fluorescent dyes lend support to the idea that CHS proteins are delivered to the cell surface via an alternative route distinct from the classical ER-Golgi body secretory pathway.
Figures





References
-
- Amnuaykanjanasin, A., and L. Epstein. 2006. A class Vb chitin synthase in Colletotrichum graminicola is localized in the growing tips of multiple cell types, in nascent septa, and during septum conversion to an end wall after hyphal breakage. Protoplasma 227:155-164. - PubMed
-
- Bartnicki-Garcia, S. 1968. Cell wall chemistry, morphogenesis and taxonomy of fungi. Annu. Rev. Microbiol. 22:87-107. - PubMed
-
- Bartnicki-Garcia, S. 2006. Chitosomes: past, present and future. FEMS Yeast Res. 6:957-965. - PubMed
-
- Bartnicki-Garcia, S. 2002. Hyphal tip growth: outstanding questions, p. 29-58. In H. D. Osiewacz (ed.), Molecular biology of fungal development. Marcel Dekker, Inc., New York, NY.
-
- Bartnicki-Garcia, S. 1990. Role of vesicles in apical growth and a new mathematical model of hyphal morphogenesis, p. 211-232. In I. B. Heath (ed.), Tip growth in plant and fungal cells. Academic Press, San Diego, CA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

