Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin
- PMID: 17644730
- PMCID: PMC1955699
- DOI: 10.1105/tpc.106.043729
Molecular interactions of a soluble gibberellin receptor, GID1, with a rice DELLA protein, SLR1, and gibberellin
Abstract
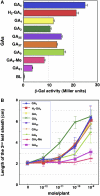
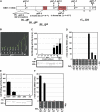
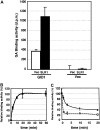
GIBBERELLIN INSENSITIVE DWARF1 (GID1) encodes a soluble gibberellin (GA) receptor that shares sequence similarity with a hormone-sensitive lipase (HSL). Previously, a yeast two-hybrid (Y2H) assay revealed that the GID1-GA complex directly interacts with SLENDER RICE1 (SLR1), a DELLA repressor protein in GA signaling. Here, we demonstrated, by pull-down and bimolecular fluorescence complementation (BiFC) experiments, that the GA-dependent GID1-SLR1 interaction also occurs in planta. GA(4) was found to have the highest affinity to GID1 in Y2H assays and is the most effective form of GA in planta. Domain analyses of SLR1 using Y2H, gel filtration, and BiFC methods revealed that the DELLA and TVHYNP domains of SLR1 are required for the GID1-SLR1 interaction. To identify the important regions of GID1 for GA and SLR1 interactions, we used many different mutant versions of GID1, such as the spontaneous mutant GID1s, N- and C-terminal truncated GID1s, and mutagenized GID1 proteins with conserved amino acids replaced with Ala. The amino acid residues important for SLR1 interaction completely overlapped the residues required for GA binding that were scattered throughout the GID1 molecule. When we plotted these residues on the GID1 structure predicted by analogy with HSL tertiary structure, many residues were located at regions corresponding to the substrate binding pocket and lid. Furthermore, the GA-GID1 interaction was stabilized by SLR1. Based on these observations, we proposed a molecular model for interaction between GA, GID1, and SLR1.
Figures






Comment in
-
GA perception and signal transduction: molecular interactions of the GA receptor GID1 with GA and the DELLA protein SLR1 in rice.Plant Cell. 2007 Jul;19(7):2095-7. doi: 10.1105/tpc.107.054916. Plant Cell. 2007. PMID: 17918309 Free PMC article. No abstract available.
References
-
- Abe, M., Kobayashi, Y., Yamamoto, S., Daimon, Y., Yamaguchi, A., Ikeda, Y., Ichinoki, H., Notaguchi, M., Goto, K., and Araki, T. (2005). FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309 1052–1056. - PubMed
-
- Cuff, J.A., Clamp, M.E., Siddiqui, A.S., Finlay, M., and Barton, G.J. (1998). Jpred: A consensus secondary structure prediction server. Bioinformatics 14 892–893. - PubMed
-
- Davies, P.J. (1995). Plant Hormones. (Dordrecht, The Netherlands: Kluwer Academic Publishers).
-
- De Simone, G., Galdiero, S., Manco, G., Lang, D., Rossi, M., and Pedone, C. (2000). A snapshot of a transition state analogue of a novel thermophilic esterase belonging to the subfamily of mammalian hormone-sensitive lipase. J. Mol. Biol. 303 761–771. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

