In vivo and in vitro effects of pilocarpine: relevance to ictogenesis
- PMID: 17645533
- PMCID: PMC3900294
- DOI: 10.1111/j.1528-1167.2007.01185.x
In vivo and in vitro effects of pilocarpine: relevance to ictogenesis
Abstract
Objectives: A common experimental model of status epilepticus (SE) utilizes intraperitoneal administration of the cholinergic agonist pilocarpine preceded by methyl-scopolamine treatment. Currently, activation of cholinergic neurons is recognized as the only factor triggering pilocarpine SE. However, cholinergic receptors are also widely distributed systemically and pretreatment with methyl-scopolamine may not be sufficient to counteract the effects of systemically injected pilocarpine. The extent of such peripheral events and the contribution to SE are unknown and the possibility that pilocarpine also induces SE by peripheral actions is yet untested.
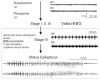
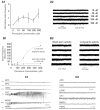
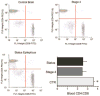
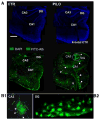
Methods: We measured in vivo at onset of SE: brain and blood pilocarpine levels, blood-brain barrier (BBB) permeability, T-lymphocyte activation and serum levels of IL-1beta and TNF-alpha. The effects of pilocarpine on neuronal excitability was assessed in vitro on hippocampal slices or whole guinea pig brain preparations in presence of physiologic or elevated [K+](out).
Results: Pilocarpine blood and brain levels at SE were 1400 +/- 200 microM and 200 +/- 80 microM, respectively. In vivo, after pilocarpine injection, increased serum IL-1beta, decreased CD4:CD8 T-lymphocyte ratios and focal BBB leakage were observed. In vitro, pilocarpine failed to exert significant synchronized epileptiform activity when applied at concentrations identical or higher to levels measured in vivo. Intense electrographic seizure-like events occurred only in the copresence of levels of K+ (6 mM) mimicking BBB leakage.
Conclusions: Early systemic events increasing BBB permeability may promote entry of cofactors (e. g. K+) into the brain leading to pilocarpine-induced SE. Disturbance of brain homeostasis represents an etiological factor contributing to pilocarpine seizures.
Figures




Similar articles
-
Antagonism of peripheral inflammation reduces the severity of status epilepticus.Neurobiol Dis. 2009 Feb;33(2):171-81. doi: 10.1016/j.nbd.2008.10.002. Epub 2008 Oct 28. Neurobiol Dis. 2009. PMID: 19010416 Free PMC article.
-
Acute induction of epileptiform discharges by pilocarpine in the in vitro isolated guinea-pig brain requires enhancement of blood-brain barrier permeability.Neuroscience. 2008 Jan 2;151(1):303-12. doi: 10.1016/j.neuroscience.2007.10.037. Epub 2007 Nov 12. Neuroscience. 2008. PMID: 18082973 Free PMC article.
-
Comparative profile of refractory status epilepticus models following exposure of cholinergic agents pilocarpine, DFP, and soman.Neuropharmacology. 2021 Jun 15;191:108571. doi: 10.1016/j.neuropharm.2021.108571. Epub 2021 Apr 18. Neuropharmacology. 2021. PMID: 33878303 Free PMC article.
-
Development of a rat pilocarpine model of seizure/status epilepticus that mimics chemical warfare nerve agent exposure.Toxicol Ind Health. 2006 Jul;22(6):255-66. doi: 10.1191/0748233706th268oa. Toxicol Ind Health. 2006. PMID: 16924957
-
A new derivative of valproic acid amide possesses a broad-spectrum antiseizure profile and unique activity against status epilepticus and organophosphate neuronal damage.Epilepsia. 2012 Jan;53(1):134-46. doi: 10.1111/j.1528-1167.2011.03338.x. Epub 2011 Dec 9. Epilepsia. 2012. PMID: 22150444 Free PMC article.
Cited by
-
The radial organization of neuronal primary cilia is acutely disrupted by seizure and ischemic brain injury.Front Biol (Beijing). 2017 Apr;12(2):124-138. doi: 10.1007/s11515-017-1447-1. Epub 2017 Mar 8. Front Biol (Beijing). 2017. PMID: 28473847 Free PMC article.
-
Novel test strategies for in vitro seizure liability assessment.Expert Opin Drug Metab Toxicol. 2021 Aug;17(8):923-936. doi: 10.1080/17425255.2021.1876026. Epub 2021 Feb 17. Expert Opin Drug Metab Toxicol. 2021. PMID: 33595380 Free PMC article. Review.
-
Loss of Long-Term Potentiation at Hippocampal Output Synapses in Experimental Temporal Lobe Epilepsy.Front Mol Neurosci. 2020 Aug 28;13:143. doi: 10.3389/fnmol.2020.00143. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32982687 Free PMC article.
-
Somatostatin type-2 receptor activation inhibits glutamate release and prevents status epilepticus.Neurobiol Dis. 2013 Jun;54:94-104. doi: 10.1016/j.nbd.2013.02.015. Epub 2013 Mar 5. Neurobiol Dis. 2013. PMID: 23473742 Free PMC article.
-
Blood-brain barrier dysfunction in epileptogenesis of the temporal lobe.Epilepsy Res Treat. 2011;2011:143908. doi: 10.1155/2011/143908. Epub 2011 Jun 7. Epilepsy Res Treat. 2011. PMID: 22937228 Free PMC article.
References
-
- Arzt ES, Fernandez-Castelo S, Diaz A, Finkielman S, Nahmod VE. The muscarinic agonist pilocarpine inhibits DNA and interferon-gamma synthesis in peripheral blood mononuclear cells. Int J Immunopharmacol. 1989;11:275–281. - PubMed
-
- Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. - PubMed
-
- Bawden HN, Racine RJ. Effects of bilateral kindling or bilateral sub-threshold stimulation of the amygdala or septum on muricide, ranacide, intraspecific aggression and passive avoidance in the rat. Physiol Behav. 1979;22:115–123. - PubMed
-
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci. 2003;17:1403–1410. - PubMed
-
- Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

