Targeting of Sna3p to the endosomal pathway depends on its interaction with Rsp5p and multivesicular body sorting on its ubiquitylation
- PMID: 17645729
- PMCID: PMC2171029
- DOI: 10.1111/j.1600-0854.2007.00610.x
Targeting of Sna3p to the endosomal pathway depends on its interaction with Rsp5p and multivesicular body sorting on its ubiquitylation
Abstract
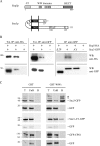
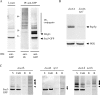
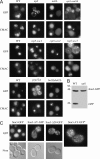
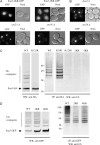
Rsp5p is an ubiquitin (Ub)-protein ligase of the Nedd4 family that carries WW domains involved in interaction with PPXY-containing proteins. It plays a key role at several stages of intracellular trafficking, such as Ub-mediated internalization of endocytic cargoes and Ub-mediated sorting of membrane proteins to internal vesicles of multivesicular bodies (MVBs), a process that is crucial for their subsequent targeting to the vacuolar lumen. Sna3p is a membrane protein previously described as an Ub-independent MVB cargo, but proteomic studies have since shown it to be an ubiquitylated protein. Sna3p carries a PPXY motif. We observed that this motif mediates its interaction with Rsp5p WW domains. Mutation of either the Sna3p PPXY motif or the Rsp5p WW3 domain or reduction in the amounts of Rsp5 results in the mistargeting of Sna3p to multiple mobile vesicles and prevents its sorting to the endosomal pathway. This sorting defect appears to occur prior to the defect displayed in rsp5 mutants by other MVB cargoes, which are correctly sorted to the endosomal pathway but missorted to the vacuolar membrane instead of the vacuolar lumen. Sna3p is polyubiquitylated on one target lysine, and a mutant Sna3p lacking its target lysine displays defective MVB sorting. Sna3p undergoes Rsp5-dependent polyubiquitylation, with K63-linked Ub chains.
Figures

Similar articles
-
Direct binding to Rsp5p regulates ubiquitination-independent vacuolar transport of Sna3p.Mol Biol Cell. 2007 May;18(5):1781-9. doi: 10.1091/mbc.e06-10-0887. Epub 2007 Mar 1. Mol Biol Cell. 2007. PMID: 17332499 Free PMC article.
-
The ubiquitin ligase Rsp5p is required for modification and sorting of membrane proteins into multivesicular bodies.Traffic. 2004 May;5(5):383-92. doi: 10.1111/j.1398-9219.2004.00183.x. Traffic. 2004. PMID: 15086787
-
Versatile role of the yeast ubiquitin ligase Rsp5p in intracellular trafficking.Biochem Soc Trans. 2008 Oct;36(Pt 5):791-6. doi: 10.1042/BST0360791. Biochem Soc Trans. 2008. PMID: 18793138 Review.
-
Characterization of multiple multivesicular body sorting determinants within Sna3: a role for the ubiquitin ligase Rsp5.Mol Biol Cell. 2007 Feb;18(2):707-20. doi: 10.1091/mbc.e06-08-0680. Epub 2006 Dec 20. Mol Biol Cell. 2007. PMID: 17182849 Free PMC article.
-
The ubiquitin code of yeast permease trafficking.Trends Cell Biol. 2010 Apr;20(4):196-204. doi: 10.1016/j.tcb.2010.01.004. Trends Cell Biol. 2010. PMID: 20138522 Review.
Cited by
-
Identification of human gene products containing Pro-Pro-x-Tyr (PY) motifs that enhance glutathione and endocytotic marker uptake in yeast.Cell Physiol Biochem. 2010;25(2-3):293-306. doi: 10.1159/000276570. Epub 2010 Jan 12. Cell Physiol Biochem. 2010. PMID: 20110690 Free PMC article.
-
A perturbed ubiquitin landscape distinguishes between ubiquitin in trafficking and in proteolysis.Mol Cell Proteomics. 2011 May;10(5):M111.009753. doi: 10.1074/mcp.M111.009753. Epub 2011 Mar 22. Mol Cell Proteomics. 2011. PMID: 21427232 Free PMC article.
-
Structural insights into the catalysis and regulation of E3 ubiquitin ligases.Nat Rev Mol Cell Biol. 2016 Oct;17(10):626-42. doi: 10.1038/nrm.2016.91. Epub 2016 Aug 3. Nat Rev Mol Cell Biol. 2016. PMID: 27485899 Free PMC article. Review.
-
The ubiquitin-proteasome system regulates mitochondrial intermembrane space proteins.Mol Cell Biol. 2013 Jun;33(11):2136-48. doi: 10.1128/MCB.01579-12. Epub 2013 Mar 18. Mol Cell Biol. 2013. PMID: 23508107 Free PMC article.
-
Regulation of the RSP5 ubiquitin ligase by an intrinsic ubiquitin-binding site.J Biol Chem. 2009 May 1;284(18):12071-9. doi: 10.1074/jbc.M901106200. Epub 2009 Feb 27. J Biol Chem. 2009. PMID: 19252184 Free PMC article.
References
-
- Dupre S, Urban-Grimal D, Haguenauer-Tsapis R. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim Biophys Acta. 2004;1695:89–111. - PubMed
-
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. - PubMed
-
- Soetens O, De Craene JO, Andre B. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J Biol Chem. 2001;276:43949–43957. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

