Involvement of cyclooxygenase-2 in the tumor site-dependent production of parathyroid hormone-related protein in colon 26 carcinoma
- PMID: 17645771
- PMCID: PMC11159920
- DOI: 10.1111/j.1349-7006.2007.00568.x
Involvement of cyclooxygenase-2 in the tumor site-dependent production of parathyroid hormone-related protein in colon 26 carcinoma
Abstract
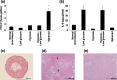
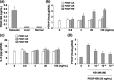
It has been shown that in the mouse colon 26 tumor model, tumors grown in the subcutis (subcutis colon 26) caused early onset of cachectic syndromes, whereas those in the liver (liver colon 26) did not. Both interleukin (IL)-6 and parathyroid hormone-related protein (PTHrP) were involved in the development of cachectic syndromes in this tumor model. However, whether expression of PTHrP and IL-6 is differently regulated in the tumor microenvironment is unclear. In the present study, culturing the colon 26 cells under different conditions in vitro revealed that IL-6 production was increased by monolayer culture under a low-glucose condition but not by spheroid culture. In contrast, PTHrP production was increased by spheroid culture but not by monolayer culture, even under a low-glucose condition. Gene expression profiling revealed that the expression of cyclooxygenase (COX)-2 was up-regulated in both subcutis colon 26 and spheroid cultures, and that COX-2 inhibitor NS-398 suppressed PTHrP production in spheroid cultures. Furthermore, administration of NS-398 decreased the PTHrP level without affecting the tumor growth in mice bearing subcutis colon 26. These results demonstrate that production of PTHrP and IL-6 largely depends on the microenvironments in which tumors are developed or metastasized and that up-regulation of COX-2 in a necrobiotic environment leads to PTHrP production, thereby causing cachectic syndromes.
Figures




Similar articles
-
Capecitabine improves cancer cachexia and normalizes IL-6 and PTHrP levels in mouse cancer cachexia models.Cancer Chemother Pharmacol. 2007 May;59(6):807-15. doi: 10.1007/s00280-006-0338-y. Epub 2006 Sep 29. Cancer Chemother Pharmacol. 2007. PMID: 17009035
-
Autocrine vascular endothelial growth factor/vascular endothelial growth factor receptor-2 growth pathway represents a cyclooxygenase-2-independent target for the cyclooxygenase-2 inhibitor NS-398 in colon cancer cells.Oncology. 2005;68(2-3):204-11. doi: 10.1159/000086775. Epub 2005 Jul 7. Oncology. 2005. PMID: 16015035
-
Regulation of the cellular expression of secretory and cytosolic phospholipases A2, and cyclooxygenase-2 by peptide growth factors.Biochim Biophys Acta. 1998 May 27;1403(1):47-56. doi: 10.1016/s0167-4889(98)00029-9. Biochim Biophys Acta. 1998. PMID: 9622592
-
[Effects of selective cyclooxygenase-2 inhibitor NS-398 on 5-fluorouracil chemotherapy and progression of colon cells: an experimental study].Zhonghua Yi Xue Za Zhi. 2004 Apr 2;84(7):583-6. Zhonghua Yi Xue Za Zhi. 2004. PMID: 15144595 Chinese.
-
Treatment of malignancy-associated hypercalcemia and cachexia with humanized anti-parathyroid hormone-related protein antibody.Semin Oncol. 2003 Oct;30(5 Suppl 16):167-73. doi: 10.1053/j.seminoncol.2003.08.019. Semin Oncol. 2003. PMID: 14613038 Review.
Cited by
-
A Diet Rich in Fish Oil and Leucine Ameliorates Hypercalcemia in Tumour-Induced Cachectic Mice.Int J Mol Sci. 2019 Oct 9;20(20):4978. doi: 10.3390/ijms20204978. Int J Mol Sci. 2019. PMID: 31600911 Free PMC article.
-
Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia.J Mol Med (Berl). 2010 Jan;88(1):85-92. doi: 10.1007/s00109-009-0547-z. Epub 2009 Oct 3. J Mol Med (Berl). 2010. PMID: 19802504 Clinical Trial.
-
Brassinin alleviates cancer cachexia by suppressing diverse inflammatory mechanisms in mice.MedComm (2020). 2024 May 28;5(6):e558. doi: 10.1002/mco2.558. eCollection 2024 Jun. MedComm (2020). 2024. PMID: 38807976 Free PMC article.
References
-
- Tisdale MJ. Biomedicine protein loss in cancer cachexia. Science 2000; 289: 2293–4. - PubMed
-
- Argiles JM, Alvarez B, Lopez‐Soriano FJ. The metabolic basis of cancer cachexia. Med Res Rev 1997; 17: 477–98. - PubMed
-
- Onuma E, Tsunenari T, Saito H, Sato K, Yamada‐Okabe H, Ogata E. Parathyroid hormone‐related protein (PTHrP) as a causative factor of cancer‐associated wasting: Possible involvement of PTHrP in the repression of locomotor activity in rats bearing human tumor xenografts. Int J Cancer 2005; 116: 471–8. - PubMed
-
- Takahashi S, Hakuta M, Aiba K et al . Elevation of circulating plasma cytokines in cancer patients with high plasma parathyroid hormone‐related protein levels. Endocr Relat Cancer 2003; 10: 403–7. - PubMed
-
- Deans C, Wigmore S, Paterson‐Brown S, Black J, Ross J, Fearon CHK. Serum parathyroid hormone‐related peptide is associated with systemic inflammation and adverse prognosis in gastroesophageal carcinoma. Cancer 2005; 103: 1810–18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

