Stress signaling in cancer
- PMID: 17645775
- PMCID: PMC11159157
- DOI: 10.1111/j.1349-7006.2007.00551.x
Stress signaling in cancer
Abstract
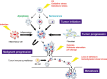
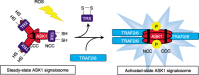
The human body is continuously exposed to a wide variety of physical, chemical, and biological stress stimuli from both the external and internal environments. In order to adapt to or resist stress, cells are equipped with multiple signaling systems, which elicit a wide range of stress responses. Stress signaling also operates to eliminate cells with severe stress-induced damage through the induction of apoptosis. Once stress signaling is compromised in certain adverse conditions, however, cells exhibit aberrant responses to stress, which can eventually cause various diseases including cancer. In the present review, the authors focus on the current understanding of the critical linkage between stress signaling and cancer.
Figures



Similar articles
-
ASK family and cancer.Adv Biol Regul. 2017 Dec;66:72-84. doi: 10.1016/j.jbior.2017.05.003. Epub 2017 May 20. Adv Biol Regul. 2017. PMID: 28552579 Review.
-
Mitogen-activated protein kinases in mammalian oxidative stress responses.Antioxid Redox Signal. 2011 Jul 1;15(1):205-18. doi: 10.1089/ars.2010.3733. Epub 2011 Apr 8. Antioxid Redox Signal. 2011. PMID: 21050144 Review.
-
Cancer, Oxidative Stress, and Metastasis.Cold Spring Harb Symp Quant Biol. 2016;81:163-175. doi: 10.1101/sqb.2016.81.030791. Epub 2017 Jan 12. Cold Spring Harb Symp Quant Biol. 2016. PMID: 28082378 Review.
-
Stress as an intercellular signal: the emergence of stress-associated molecular patterns (SAMP).Antioxid Redox Signal. 2009 Oct;11(10):2621-9. doi: 10.1089/ars.2009.2377. Antioxid Redox Signal. 2009. PMID: 19320597 Review.
-
Transcriptional responses to oxidative stress: pathological and toxicological implications.Pharmacol Ther. 2010 Mar;125(3):376-93. doi: 10.1016/j.pharmthera.2009.11.004. Epub 2009 Nov 27. Pharmacol Ther. 2010. PMID: 19945483 Review.
Cited by
-
Fatty acid synthase as a potential therapeutic target in cancer.Future Oncol. 2010 Apr;6(4):551-62. doi: 10.2217/fon.10.11. Future Oncol. 2010. PMID: 20373869 Free PMC article. Review.
-
ASK1 facilitates tumor metastasis through phosphorylation of an ADP receptor P2Y12 in platelets.Cell Death Differ. 2017 Dec;24(12):2066-2076. doi: 10.1038/cdd.2017.114. Epub 2017 Jul 28. Cell Death Differ. 2017. PMID: 28753204 Free PMC article.
-
In vivo gene manipulation reveals the impact of stress-responsive MAPK pathways on tumor progression.Cancer Sci. 2015 Jul;106(7):785-96. doi: 10.1111/cas.12676. Epub 2015 May 25. Cancer Sci. 2015. PMID: 25880821 Free PMC article. Review.
-
ASK1 and ASK2 differentially regulate the counteracting roles of apoptosis and inflammation in tumorigenesis.EMBO J. 2009 Apr 8;28(7):843-53. doi: 10.1038/emboj.2009.32. Epub 2009 Feb 12. EMBO J. 2009. PMID: 19214184 Free PMC article.
-
De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy.Br J Cancer. 2009 May 5;100(9):1369-72. doi: 10.1038/sj.bjc.6605007. Epub 2009 Apr 7. Br J Cancer. 2009. PMID: 19352381 Free PMC article. Review.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. - PubMed
-
- Karin M, Greten FR. NF‐kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005; 5: 749–59. - PubMed
-
- Maeda S, Kamata H, Luo JL et al . IKKbeta couples hepatocyte death to cytokine‐driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005; 121: 977–90. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005; 120: 513–22. - PubMed
-
- Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 2004; 4: 966–77. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

