NeuA sialic acid O-acetylesterase activity modulates O-acetylation of capsular polysaccharide in group B Streptococcus
- PMID: 17646166
- PMCID: PMC2588433
- DOI: 10.1074/jbc.M700340200
NeuA sialic acid O-acetylesterase activity modulates O-acetylation of capsular polysaccharide in group B Streptococcus
Abstract
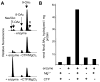
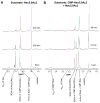
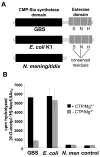
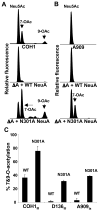
Group B Streptococcus (GBS) is a common cause of neonatal sepsis and meningitis. A major GBS virulence determinant is its sialic acid (Sia)-capped capsular polysaccharide. Recently, we discovered the presence and genetic basis of capsular Sia O-acetylation in GBS. We now characterize a GBS Sia O-acetylesterase that modulates the degree of GBS surface O-acetylation. The GBS Sia O-acetylesterase operates cooperatively with the GBS CMP-Sia synthetase, both part of a single polypeptide encoded by the neuA gene. NeuA de-O-acetylation of free 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac(2)) was enhanced by CTP and Mg(2+), the substrate and co-factor, respectively, of the N-terminal GBS CMP-Sia synthetase domain. In contrast, the homologous bifunctional NeuA esterase from Escherichia coli K1 did not display cofactor dependence. Further analyses showed that in vitro, GBS NeuA can operate via two alternate enzymatic pathways: de-O-acetylation of Neu5,9Ac(2) followed by CMP activation of Neu5Ac or activation of Neu5,9Ac(2) followed by de-O-acetylation of CMP-Neu5,9Ac(2). Consistent with in vitro esterase assays, genetic deletion of GBS neuA led to accumulation of intracellular O-acetylated Sias, and overexpression of GBS NeuA reduced O-acetylation of Sias on the bacterial surface. Site-directed mutagenesis of conserved asparagine residue 301 abolished esterase activity but preserved CMP-Sia synthetase activity, as evidenced by hyper-O-acetylation of capsular polysaccharide Sias on GBS expressing only the N301A NeuA allele. These studies demonstrate a novel mechanism regulating the extent of capsular Sia O-acetylation in intact bacteria and provide a genetic strategy for manipulating GBS O-acetylation in order to explore the role of this modification in GBS pathogenesis and immunogenicity.
Figures
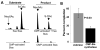
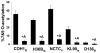


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

