Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA
- PMID: 17646382
- PMCID: PMC2099242
- DOI: 10.1128/MCB.00815-07
Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA
Abstract
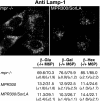
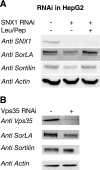
SorLA/LR11 (250 kDa) is the largest and most composite member of the Vps10p-domain receptors, a family of type 1 proteins preferentially expressed in neuronal tissue. SorLA binds several ligands, including neurotensin, platelet-derived growth factor-bb, and lipoprotein lipase, and via complex-formation with the amyloid precursor protein it downregulates generation of Alzheimer's disease-associated Abeta-peptide. The receptor is mainly located in vesicles, suggesting a function in protein sorting and transport. Here we examined SorLA's trafficking using full-length and chimeric receptors and find that its cytoplasmic tail mediates efficient Golgi body-endosome transport, as well as AP-2 complex-dependent endocytosis. Functional sorting sites were mapped to an acidic cluster-dileucine-like motif and to a GGA binding site in the C terminus. Experiments in permanently or transiently AP-1 mu1-chain-deficient cells established that the AP-1 adaptor complex is essential to SorLA's transport between Golgi membranes and endosomes. Our results further implicate the GGA proteins in SorLA trafficking and provide evidence that SNX1 and Vps35, as parts of the retromer complex or possibly in a separate context, are engaged in retraction of the receptor from endosomes.
Figures


Similar articles
-
The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein.EMBO J. 2001 May 1;20(9):2180-90. doi: 10.1093/emboj/20.9.2180. EMBO J. 2001. PMID: 11331584 Free PMC article.
-
Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein.Proc Natl Acad Sci U S A. 2005 Sep 20;102(38):13461-6. doi: 10.1073/pnas.0503689102. Epub 2005 Sep 7. Proc Natl Acad Sci U S A. 2005. PMID: 16174740 Free PMC article.
-
Calnuc binds to LRP9 and affects its endosomal sorting.Traffic. 2009 Aug;10(8):1098-114. doi: 10.1111/j.1600-0854.2009.00933.x. Epub 2009 May 30. Traffic. 2009. PMID: 19497050
-
Sorting receptor SORLA--a trafficking path to avoid Alzheimer disease.J Cell Sci. 2013 Jul 1;126(Pt 13):2751-60. doi: 10.1242/jcs.125393. Epub 2013 Jun 26. J Cell Sci. 2013. PMID: 23813966 Review.
-
sorLA: sorting out APP.Mol Interv. 2006 Apr;6(2):74-6, 58. doi: 10.1124/mi.6.2.4. Mol Interv. 2006. PMID: 16565469 Review.
Cited by
-
The Vps10p-domain receptor family.Cell Mol Life Sci. 2009 Aug;66(16):2677-89. doi: 10.1007/s00018-009-0043-1. Epub 2009 May 12. Cell Mol Life Sci. 2009. PMID: 19434368 Free PMC article. Review.
-
Toward Understanding the Molecular Role of SNX27/Retromer in Human Health and Disease.Front Cell Dev Biol. 2021 Apr 15;9:642378. doi: 10.3389/fcell.2021.642378. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937239 Free PMC article. Review.
-
SorLA and CLC:CLF-1-dependent Downregulation of CNTFRα as Demonstrated by Western Blotting, Inhibition of Lysosomal Enzymes, and Immunocytochemistry.J Vis Exp. 2017 Jan 6;(119):55019. doi: 10.3791/55019. J Vis Exp. 2017. PMID: 28117780 Free PMC article.
-
Retromer.Curr Opin Cell Biol. 2008 Aug;20(4):427-36. doi: 10.1016/j.ceb.2008.03.009. Epub 2008 May 9. Curr Opin Cell Biol. 2008. PMID: 18472259 Free PMC article. Review.
-
Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis.Neuron. 2008 Nov 26;60(4):534-42. doi: 10.1016/j.neuron.2008.11.007. Neuron. 2008. PMID: 19038212 Free PMC article. Review.
References
-
- Andersen, O. M., J. Reiche, V. Schmidt, M. Gotthardt, R. Spoelgen, J. Behlke, C. A. von Arnim, T. Breiderhoff, P. Jansen, X. Wu, K. R. Bales, R. Cappai, C. L. Masters, J. Gliemann, E. J. Mufson, B. T. Hyman, S. M. Paul, A. Nykjaer, and T. E. Willnow. 2005. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 102:13461-13466. - PMC - PubMed
-
- Andersen, O. M., V. Schmidt, R. Spoelgen, J. Gliemann, J. Behlke, D. Galatis, W. J. McKinstry, M. W. Parker, C. L. Masters, B. T. Hyman, R. Cappai, and T. E. Willnow. 2006. Molecular dissection of the interaction between amyloid precursor protein and its neuronal trafficking receptor SorLA/LR11. Biochemistry 45:2618-2628. - PubMed
-
- Bergeland, T., J. Widerberg, O. Bakke, and T. W. Nordeng. 2001. Mitotic partitioning of endosomes and lysosomes. Curr. Biol. 11:644-651. - PubMed
-
- Bonifacino, J. S., and R. Rojas. 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell. Biol. 7:568-579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
