Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells
- PMID: 17646404
- PMCID: PMC2118686
- DOI: 10.1084/jem.20062373
Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells
Abstract
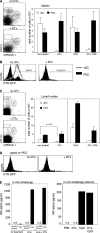
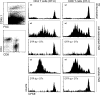
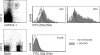
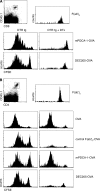
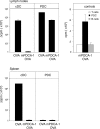
Plasmacytoid dendritic cells (PDCs) play a pivotal role as cytokine-secreting accessory cells in the antimicrobial immune defense. In contrast, the capacity of PDCs to act as antigen-presenting cells in naive T cell priming remains unclear. By studying T cell responses in mice that lack conventional DCs (cDCs), and by the use of a PDC-specific antigen-targeting strategy, we show that PDCs can initiate productive naive CD4(+) T cell responses in lymph nodes, but not in the spleen. PDC-triggered CD4(+) T cell responses differed from cDC-driven responses in that they were not associated with concomitant CD8(+) T cell priming. Our results establish PDCs as a bona fide DC subset that initiates unique CD4(+) Th cell-dominated primary immune responses.
Figures


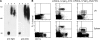
References
-
- Liu, Y.J. 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23:275–306. - PubMed
-
- Kawai, T., S. Sato, K.J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068. - PubMed
-
- Lennert, K., and W. Remmele. Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes. 1958. Acta Haematol. 19:99–113. - PubMed
-
- Trinchieri, G., and D. Santoli. 1978. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J. Exp. Med. 147:1314–1333. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

