FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors
- PMID: 17646409
- PMCID: PMC2118656
- DOI: 10.1084/jem.20070876
FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors
Abstract
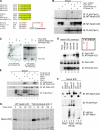
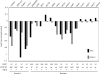
gamma-secretase inhibitors (GSIs) can block NOTCH receptor signaling in vitro and therefore offer an attractive targeted therapy for tumors dependent on deregulated NOTCH activity. To clarify the basis for GSI resistance in T cell acute lymphoblastic leukemia (T-ALL), we studied T-ALL cell lines with constitutive expression of the NOTCH intracellular domain (NICD), but that lacked C-terminal truncating mutations in NOTCH1. Each of the seven cell lines examined and 7 of 81 (8.6%) primary T-ALL samples harbored either a mutation or homozygous deletion of the gene FBW7, a ubiquitin ligase implicated in NICD turnover. Indeed, we show that FBW7 mutants cannot bind to the NICD and define the phosphodegron region of the NICD required for FBW7 binding. Although the mutant forms of FBW7 were still able to bind to MYC, they do not target it for degradation, suggesting that stabilization of both NICD and its principle downstream target, MYC, may contribute to transformation in leukemias with FBW7 mutations. In addition, we show that all seven leukemic cell lines with FBW7 mutations were resistant to the MRK-003 GSI. Most of these resistant lines also failed to down-regulate the mRNA levels of the NOTCH targets MYC and DELTEX1 after treatment with MRK-003, implying that residual NOTCH signaling in T-ALLs with FBW7 mutations contributes to GSI resistance.
Figures




References
-
- Wilson, A., and F. Radtke. 2006. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 580:2860–2868. - PubMed
-
- Dievart, A., N. Beaulieu, and P. Jolicoeur. 1999. Involvement of Notch1 in the development of mouse mammary tumors. Oncogene. 18:5973–5981. - PubMed
-
- Hallahan, A.R., J.I. Pritchard, S. Hansen, M. Benson, J. Stoeck, B.A. Hatton, T.L. Russell, R.G. Ellenbogen, I.D. Bernstein, P.A. Beachy, and J.M. Olson. 2004. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 64:7794–7800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

