Sumoylation in axons triggers retrograde transport of the RNA-binding protein La
- PMID: 17646655
- PMCID: PMC1937566
- DOI: 10.1073/pnas.0611562104
Sumoylation in axons triggers retrograde transport of the RNA-binding protein La
Abstract
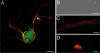
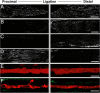
A surprisingly large population of mRNAs has been shown to localize to sensory axons, but few RNA-binding proteins have been detected in these axons. These axonal mRNAs include several potential binding targets for the La RNA chaperone protein. La is transported into axonal processes in both culture and peripheral nerve. Interestingly, La is posttranslationally modified in sensory neurons by sumoylation. In axons, small ubiquitin-like modifying polypeptides (SUMO)-La interacts with dynein, whereas native La interacts with kinesin. Lysine 41 is required for sumoylation, and sumoylation-incompetent La(K41R) shows only anterograde transport, whereas WT La shows both anterograde and retrograde transport in axons. Thus, sumoylation of La determines the directionality of its transport within the axonal compartment, with SUMO-La likely recycling to the cell body.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

