Elevated NF-kappaB responses and FLIP levels in leukemic but not normal lymphocytes: reduction by salicylate allows TNF-induced apoptosis
- PMID: 17646662
- PMCID: PMC1937545
- DOI: 10.1073/pnas.0701437104
Elevated NF-kappaB responses and FLIP levels in leukemic but not normal lymphocytes: reduction by salicylate allows TNF-induced apoptosis
Abstract
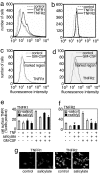
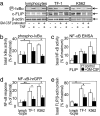
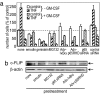
As its name suggests, tumor necrosis factor (TNF) is known to induce cytotoxicity in a wide variety of tumor cells and cell lines. However, its use as a chemotherapeutic drug has been limited by its deleterious side effects of systemic shock and widespread inflammatory responses. Some nonsteroidal antiinflammatory drugs, such as sodium salicylate, have been shown to have a chemopreventive role in certain forms of cancer. Here, we reveal that sodium salicylate selectively enhances the apoptotic effects of TNF in human erythroleukemia cells but does not affect primary human lymphocytes or monocytes. Sodium salicylate did not affect the intracellular distribution of TNF receptors (TNFRs) but stimulated cell surface TNFR2 shedding. Erythroleukemia cells were shown to possess markedly greater basal NF-kappaB responses and elevated Fas-associated protein with death domain-like IL-1 converting enzyme (FLIP) levels. Sodium salicylate achieved its effects by reducing the elevated NF-kappaB responsiveness and FLIP levels and restoring the apoptotic response of TNF rather than the proliferative/proinflammatory effects of the cytokine in these cancer cells. Inhibition of NF-kappaB or FLIP levels in human erythroleukemia cells by pharmacological or molecular-biological means also resulted in switching the character of these cells from a TNF-responsive proliferative phenotype into an apoptotic one. These findings expose that the enhanced proliferative nature of human leukemia cells is caused by elevated NF-kappaB and FLIP responses and basal levels, reversible by sodium salicylate to allow greater apoptotic responsiveness of cytotoxic stimuli such as TNF. Such findings provide insight into the molecular mechanisms by which human leukemia cells can switch from a proliferative into an apoptotic phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
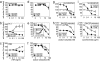

References
-
- Fiers W. FEBS Lett. 1991;285:199–212. - PubMed
-
- Berkova N, Lemay A, Korobko V, Shingarova L, Sagaidak L, Goupil S. Cancer Detection Prevention. 1999;23:1–7. - PubMed
-
- MacEwan DJ. Cell Signalling. 2002;14:477–492. - PubMed
-
- Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbararnaiah K. Lancet Oncol. 2001;2:544–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

