The chemical component of the mixed GF-TTMn synapse in Drosophila melanogaster uses acetylcholine as its neurotransmitter
- PMID: 17650116
- PMCID: PMC1974813
- DOI: 10.1111/j.1460-9568.2007.05686.x
The chemical component of the mixed GF-TTMn synapse in Drosophila melanogaster uses acetylcholine as its neurotransmitter
Abstract
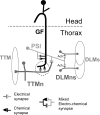
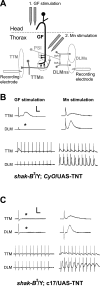
The largest central synapse in adult Drosophila is a mixed electro-chemical synapse whose gap junctions require the product of the shaking-B (shak-B) gene. Shak-B(2) mutant flies lack gap junctions at this synapse, which is between the giant fibre (GF) and the tergotrochanteral motor neuron (TTMn), but it still exhibits a long latency response upon GF stimulation. We have targeted the expression of the light chain of tetanus toxin to the GF, to block chemical transmission, in shak-B(2) flies. The long latency response in the tergotrochanteral muscle (TTM) was abolished indicating that the chemical component of the synapse mediates this response. Attenuation of GAL4-mediated labelling by a cha-GAL80 transgene, reveals the GF to be cholinergic. We have used a temperature-sensitive allele of the choline acetyltransferase gene (cha(ts2)) to block cholinergic synapses in adult flies and this also abolished the long latency response in shak-B(2) flies. Taken together the data provide evidence that both components of this mixed synapse are functional and that the chemical neurotransmitter between the GF and the TTMn is acetylcholine. Our findings show that the two components of this synapse can be separated to allow further studies into the mechanisms by which mixed synapses are built and function.
Figures



References
-
- Allen MJ, Drummond JA, Moffat KG. Development of the giant fiber neuron of Drosophila melanogaster. JCompNeurol. 1998;397:519–531. - PubMed
-
- Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fibre system. SeminCellDevBiol. 2006;17:31–41. - PubMed
-
- Allen MJ, Shan XL, Murphey RK. A role for Drosophila Drac1 in neurite outgrowth and synaptogenesis in the giant fiber system. MolCellNeurosci. 2000;16:754–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

