Intramyocellular lipid kinetics and insulin resistance
- PMID: 17650308
- PMCID: PMC1971250
- DOI: 10.1186/1476-511X-6-18
Intramyocellular lipid kinetics and insulin resistance
Abstract
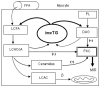
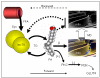
More than fifteen years ago it was discovered that intramyocellular triglyceride (imcTG) content in skeletal muscle is abnormally high in conditions of lipid oversupply (e.g. high fat feeding) and, later, obesity, type 2 diabetes (T2D) and other metabolic conditions. This imcTG excess is robustly associated with muscle insulin resistance (MIR). However, to date the pathways responsible for the imcTG excess and the mechanisms underlying the imcTG-MIR correlation remain unclear. A current hypothesis is based on a backward mechanism that impaired fatty acid oxidation by skeletal muscle causes imcTG to accumulate. As such, imcTG excess is considered a marker but not a player in MIR. However, recent results from kinetic studies indicated that imcTG pool in high fat-induced obesity (HFO) model is kinetically dynamic. On one hand, imcTG synthesis is accelerated and contributes to imcTG accumulation. On the other, the turnover of imcTG is also accelerated. A hyperdynamic imcTG pool can impose dual adverse effects on glucose metabolism in skeletal muscle. It increases the release and thus the availability of fatty acids in myocytes that may promote fatty acid oxidation and suppress glucose utilization. Meanwhile, it releases abundant fatty acid products (e.g. diacylglycerol, ceramides) that impair insulin actions via signal transduction, thereby causing MIR. Thus, intramyocellular fatty acids and their products released from imcTG appear to function as a link to MIR. Accordingly, a forward mechanism is proposed that explains the imcTG-MIR correlation.
Figures
References
-
- Storlien LH, Jenkins AB, Chrisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rat. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diabetes.40.2.280. - DOI - PubMed
-
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

