Short-term prolactin administration causes expressible galactorrhea but does not affect bone turnover: pilot data for a new lactation agent
- PMID: 17650319
- PMCID: PMC1950489
- DOI: 10.1186/1746-4358-2-10
Short-term prolactin administration causes expressible galactorrhea but does not affect bone turnover: pilot data for a new lactation agent
Abstract
Background: Medications used to augment lactation increase prolactin secretion but can have intolerable side effects. We examined the biological activity of recombinant human prolactin (r-hPRL) as preliminary data for its use to augment lactation.
Methods: Healthy, non-postpartum women (n = 21) with regular menstrual cycles underwent a seven day randomized, double-blind, placebo-controlled trial of r-hPRL. Expressible galactorrhea, markers of bone turnover, calcium homeostasis and gonadal function were measured and side effects recorded.
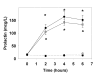
Results: Prolactin levels increased during r-hPRL administration (20.0 +/- 2.8 to 231.7 +/- 48.9 microg/L at 6 hours; p < 0.05). Five of nine participants who received r-hPRL developed expressible galactorrhea (p < 0.001). Urinary deoxypyridinoline decreased and bone specific alkaline phosphatase increased in r-hPRL and placebo groups. Menstrual cycle lengths were not altered and side effects were similar between r-hPRL and placebo groups.
Conclusion: In summary, r-hPRL can cause expressible galactorrhea. Seven days of r-hPRL administration does not adversely affect bone turnover or menstrual cyclicity. Thus, r-hPRL may be a viable option for short-term lactation augmentation.
Trial registration: Clinical Trials.gov NCT00438490.
Figures

Similar articles
-
Effects of recombinant human prolactin on breast milk composition.Pediatrics. 2011 Feb;127(2):e359-66. doi: 10.1542/peds.2010-1627. Epub 2011 Jan 24. Pediatrics. 2011. PMID: 21262884 Free PMC article. Clinical Trial.
-
Recombinant human prolactin for the treatment of lactation insufficiency.Clin Endocrinol (Oxf). 2010 Nov;73(5):645-53. doi: 10.1111/j.1365-2265.2010.03850.x. Clin Endocrinol (Oxf). 2010. PMID: 20718766 Free PMC article. Clinical Trial.
-
Inhibition of cyclic gonadotropin secretion by endogenous human prolactin.Am J Obstet Gynecol. 1975 Feb 1;121(3):375-9. doi: 10.1016/0002-9378(75)90015-0. Am J Obstet Gynecol. 1975. PMID: 1115151
-
Drugs that affect the breast and lactation.Clin Obstet Gynecol. 1975 Jun;18(2):95-111. doi: 10.1097/00003081-197506000-00006. Clin Obstet Gynecol. 1975. PMID: 1095275 Review.
-
Antipsychotics: impact on prolactin levels.Expert Opin Pharmacother. 2002 Oct;3(10):1381-91. doi: 10.1517/14656566.3.10.1381. Expert Opin Pharmacother. 2002. PMID: 12387684 Review.
Cited by
-
Effects of recombinant human prolactin on breast milk composition.Pediatrics. 2011 Feb;127(2):e359-66. doi: 10.1542/peds.2010-1627. Epub 2011 Jan 24. Pediatrics. 2011. PMID: 21262884 Free PMC article. Clinical Trial.
-
Prolactin deficiency in the context of other pituitary hormone abnormalities : Special issue: hypoprolactinemia: a neglected endocrine disorder.Rev Endocr Metab Disord. 2024 Dec;25(6):1041-1046. doi: 10.1007/s11154-024-09902-z. Epub 2024 Oct 2. Rev Endocr Metab Disord. 2024. PMID: 39356415 Free PMC article. Review.
-
A long-lasting prolactin stimulates galactopoiesis in mice.iScience. 2025 Jul 15;28(8):113112. doi: 10.1016/j.isci.2025.113112. eCollection 2025 Aug 15. iScience. 2025. PMID: 40792031 Free PMC article.
-
Recombinant human prolactin for the treatment of lactation insufficiency.Clin Endocrinol (Oxf). 2010 Nov;73(5):645-53. doi: 10.1111/j.1365-2265.2010.03850.x. Clin Endocrinol (Oxf). 2010. PMID: 20718766 Free PMC article. Clinical Trial.
References
-
- Powers NG. Slow weight gain and low milk supply in the breastfeeding dyad. Clin Perinatol. 1999;26:399–430. - PubMed
-
- Meier PP, Brown LP. State of the science: breastfeeding for mothers of low birth weight infants. Nurs Clin North Am. 1996;31:351–365. - PubMed
-
- Kauppila A, Chatelain P, Kirkinen P, Kivinen S, Ruokonen A. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J Clin Endocrinol Metab. 1987;64:309–312. - PubMed
-
- Brun del Re R, del Pozo E, de Grandi P, Friesen H, Hinselmann M, Wyss H. Prolactin inhibition and suppression of puerperal lactation by a Br-ergocryptine (CB 154) Obstet Gynecol. 1973;41:884–890. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

