Human receptor kinetics and lung tissue retention of the enhanced-affinity glucocorticoid fluticasone furoate
- PMID: 17650349
- PMCID: PMC1950704
- DOI: 10.1186/1465-9921-8-54
Human receptor kinetics and lung tissue retention of the enhanced-affinity glucocorticoid fluticasone furoate
Abstract
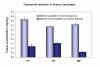
Fluticasone furoate (FF)--USAN approved name, a new topically active glucocorticoid has been recently identified. The aim of this study was to characterise the binding affinity of this compound to the human lung glucocorticoid receptor in relation to other glucocorticoids. Additionally, we sought to determine the binding behaviour of fluticasone furoate to human lung tissue. The glucocorticoid receptor binding kinetics of fluticasone furoate revealed a remarkably fast association and a slow dissociation resulting in a relative receptor affinity (RRA) of 2989 +/- 135 with reference to dexamethasone (RRA: 100 +/- 5). Thus, the RRA of FF exceeds the RRAs of all currently clinically used corticosteroids such as mometasone furoate (MF; RRA 2244), fluticasone propionate (FP; RRA 1775), ciclesonide's active metabolite (RRA 1212 - rat receptor data) or budesonide (RRA 855). FP and FF displayed pronounced retention in human lung tissue in vitro. Lowest tissue binding was found for MF. There was no indication of instability or chemical modification of FF in human lung tissue. These advantageous binding attributes may contribute to a highly efficacious profile for FF as a topical treatment for inflammatory disorders of the respiratory tract.
Figures



References
-
- Högger P. Comparison of the tissue affinity of glucocorticoids to human lung, nasal and skin tissue in vitro. Arzneimittelforschung. 2001;51:825–831. - PubMed
-
- Würthwein G, Rehder S, Rohdewald P. Lipophilicity and receptor affinity of glucocorticoids. Pharm Ztg Wiss. 1992;4:161–167.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

