Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor
- PMID: 17650501
- PMCID: PMC2913440
- DOI: 10.1074/jbc.M705669200
Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor
Abstract
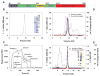
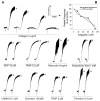
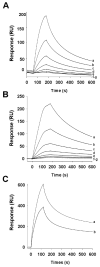
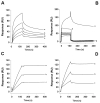
Blood-sucking arthropods have evolved a number of inhibitors of platelet aggregation and blood coagulation. In this study we have molecularly and functionally characterized aegyptin, a member of the family of 30-kDa salivary allergens from Aedes aegypti, whose function remained elusive thus far. Aegyptin displays a unique sequence characterized by glycine, glutamic acid, and aspartic acid repeats and was shown to specifically block collagen-induced human platelet aggregation and granule secretion. Plasmon resonance experiments demonstrate that aegyptin binds to collagen types I-V (K(d) approximately 1 nm) but does not interact with vitronectin, fibronectin, laminin, fibrinogen, and von Willebrand factor (vWf). In addition, aegyptin attenuates platelet adhesion to soluble or fibrillar collagen. Furthermore, aegyptin inhibits vWf interaction with collagen type III under static conditions and completely blocks platelet adhesion to collagen under flow conditions at high shear rates. Notably, aegyptin prevents collagen but not convulxin binding to recombinant glycoprotein VI. These findings suggest that aegyptin recognizes specific binding sites for glycoprotein VI, integrin alpha2beta1, and vWf, thereby preventing collagen interaction with its three major ligands. Aegyptin is a novel tool to study collagen-platelet interaction and a prototype for development of molecules with antithrombotic properties.
Figures
Similar articles
-
Simplagrin, a platelet aggregation inhibitor from Simulium nigrimanum salivary glands specifically binds to the Von Willebrand factor receptor in collagen and inhibits carotid thrombus formation in vivo.PLoS Negl Trop Dis. 2014 Jun 12;8(6):e2947. doi: 10.1371/journal.pntd.0002947. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24921659 Free PMC article.
-
Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo.FEBS J. 2010 Jan;277(2):413-27. doi: 10.1111/j.1742-4658.2009.07494.x. Epub 2009 Dec 15. FEBS J. 2010. PMID: 20015075 Free PMC article.
-
Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti.Proc Natl Acad Sci U S A. 2014 May 13;111(19):6946-51. doi: 10.1073/pnas.1404179111. Epub 2014 Apr 28. Proc Natl Acad Sci U S A. 2014. PMID: 24778255 Free PMC article.
-
Structure-activity relationships of snake toxins targeting platelet receptors, glycoprotein Ib-IX-V and glycoprotein VI.Curr Med Chem Cardiovasc Hematol Agents. 2003 Jun;1(2):143-9. doi: 10.2174/1568016033477559. Curr Med Chem Cardiovasc Hematol Agents. 2003. PMID: 15320694 Review.
-
Production and characterization of saratin, an inhibitor of von Willebrand factor-dependent platelet adhesion to collagen.Semin Thromb Hemost. 2001 Aug;27(4):337-48. doi: 10.1055/s-2001-16887. Semin Thromb Hemost. 2001. PMID: 11547356 Review.
Cited by
-
The crystal structure of the active domain of Anopheles anti-platelet protein, a powerful anti-coagulant, in complex with an antibody.J Biol Chem. 2014 Jun 6;289(23):16303-12. doi: 10.1074/jbc.M114.564526. Epub 2014 Apr 24. J Biol Chem. 2014. PMID: 24764297 Free PMC article.
-
Antibodies to Aedes aegypti D7L salivary proteins as a new serological tool to estimate human exposure to Aedes mosquitoes.Front Immunol. 2024 May 1;15:1368066. doi: 10.3389/fimmu.2024.1368066. eCollection 2024. Front Immunol. 2024. PMID: 38751433 Free PMC article.
-
Simplagrin, a platelet aggregation inhibitor from Simulium nigrimanum salivary glands specifically binds to the Von Willebrand factor receptor in collagen and inhibits carotid thrombus formation in vivo.PLoS Negl Trop Dis. 2014 Jun 12;8(6):e2947. doi: 10.1371/journal.pntd.0002947. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24921659 Free PMC article.
-
Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo.FEBS J. 2010 Jan;277(2):413-27. doi: 10.1111/j.1742-4658.2009.07494.x. Epub 2009 Dec 15. FEBS J. 2010. PMID: 20015075 Free PMC article.
-
An insight into the sialome of Glossina morsitans morsitans.BMC Genomics. 2010 Mar 30;11:213. doi: 10.1186/1471-2164-11-213. BMC Genomics. 2010. PMID: 20353571 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

