Efficacy of rifabutin-based triple therapy as second-line treatment to eradicate helicobacter pylori infection
- PMID: 17651479
- PMCID: PMC1941741
- DOI: 10.1186/1471-230X-7-31
Efficacy of rifabutin-based triple therapy as second-line treatment to eradicate helicobacter pylori infection
Abstract
Background: Rifabutin has been found to be effective in multi-resistant patients after various treatment cycles for Helicobacter pylori (HP) infection, but it has not been analysed as a second-line treatment. Therefore, we seek to compare the effectiveness of a treatment regimen including rifabutin versus conventional quadruple therapy (QT).
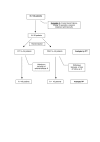
Methods: Open clinical trial, randomised and multi-centre, of two treatment protocols: A) Conventional regime -QT- (omeprazole 20 mg bid, bismuth citrate 120 mg qid, tetracycline 500 mg qid and metronidazole 500 mg tid); B) Experimental one -OAR- (omeprazole 20 mg bid, amoxicillin 1 gr bid, and rifabutin 150 mg bid), both taken orally for 7 days, in patients with HP infection for whom first-line treatment had failed. Eradication was determined by Urea Breath Test (UBT). Safety was determined by the adverse events.
Results: 99 patients were randomised, QT, n = 54; OAR, n = 45. The two groups were homogeneous. In 8 cases, treatment was suspended (6 in QT and 2 in OAR). The eradication achieved, analysed by ITT, was for QT, 38 cases (70.4%), and for OAR, 20 cases (44.4%); p = 0.009, OR = 1.58. Of the cases analysed PP, QT were 77.1%; OAR, 46.5%; p = 0.002. Adverse effects were described in 64% of the QT patients and in 44% of the OAR patients (p = 0.04).
Conclusion: A 7-day rifabutin-based triple therapy associated to amoxicillin and omeprazole at standard dose was not found to be effective as a second-line rescue therapy. The problem with quadruple therapy lies in the adverse side effects it provokes. We believe the search should continue for alternatives that are more comfortably administered and that are at least as effective, but with fewer adverse side effects.
Trial registration: Current Controlled Trials ISRCTN81058036.
Figures
References
-
- Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G, European Helicobacter Pylori Study Group (EHPSG) Current concepts in the management of Helicobacter pylori infection – the Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. - DOI - PubMed
-
- Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:1–12. - PubMed
-
- Gisbert JP, Gisbert JL, Marcos S, Gravalos RG, Carpio D, Pajares JM. Seven-day 'rescue' therapy after Helicobacter pylori treatment failure: omeprazole, bismuth, tetracycline and metronidazole vs. ranitidine bismuth citrate, tetracycline and metronidazole. Aliment Pharmacol Ther. 1999;13:1311–6. doi: 10.1046/j.1365-2036.1999.00615.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous