Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion
- PMID: 17651900
- PMCID: PMC2710388
- DOI: 10.1016/j.neuroscience.2007.04.064
Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion
Abstract
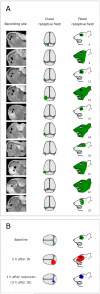
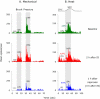
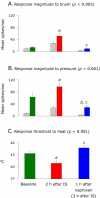
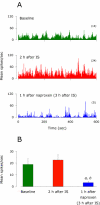
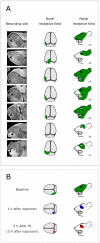
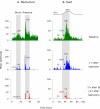
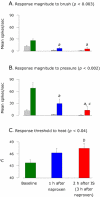
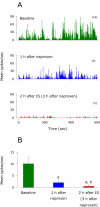
We have previously observed that migraine attacks impervious to triptan therapy were readily terminated by subsequent i.v. administration of the non-steroidal anti-inflammatory drug (NSAID) ketorolac. Since such attacks were associated with periorbital allodynia--a symptom of central sensitization--we examined whether infusion of the NSAID naproxen can block sensitization of central trigeminovascular neurons in the medullary dorsal horn, using in vivo single-unit recording in the rat. Topical exposure of the cerebral dura to inflammatory soup (IS) for 5 min resulted in a short-term burst of activity (<8 min) and a long-lasting (>120 min) neuronal hyper-responsiveness to stimulation of the dura and periorbital skin (group 1). Infusion of naproxen (1 mg/kg) 2 h after IS (group 1) brought all measures of neuronal responsiveness back to the baseline values recorded prior to IS, and depressed ongoing spontaneous activity well below baseline. When given preemptively 1 h before IS (group 2), naproxen blocked the short-term burst of activity and every long-term measure of neuronal hyper-responsiveness that was studied in the central neurons. The same preemptive treatment, however, failed to block IS-induced short-term bursts of activity in C-unit meningeal nociceptors (group 3). The results suggest that parenteral administration of naproxen, unlike triptan therapy, can exert direct inhibition over central trigeminovascular neurons in the dorsal horn. Though impractical as a routine migraine therapy, parenteral NSAID administration should be useful as a non-narcotic rescue therapy for migraine in the setting of the emergency department.
Figures


References
-
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: A race against the development of central sensitization. Ann Neurol. 2004;55:27–36. - PubMed
-
- Burstein R, Jakubowski M, Collins B. Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. - PubMed
-
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. Journal of Neurophysiology. 1998;79:964–982. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

