Lipids and lipid modifications in the regulation of membrane traffic
- PMID: 17651957
- PMCID: PMC2042035
- DOI: 10.1016/j.ceb.2007.06.003
Lipids and lipid modifications in the regulation of membrane traffic
Abstract
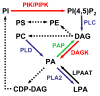
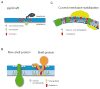
Lipids play a multitude of roles in intracellular protein transport and membrane traffic. While a large body of data implicates phosphoinositides in these processes, much less is known about other glycerophospholipids such as phosphatidic acid, diacylglycerol, and phosphatidylserine. Growing evidence suggests that these lipids may also play an important role, either by mediating protein recruitment to membranes or by directly affecting membrane dynamics. Although membrane lipids are believed to be organized in microdomains, recent advances in cellular imaging methods paired with sophisticated reporters and proteomic analysis have led to the formulation of alternative ideas regarding the characteristics and putative functions of lipid microdomains and their associated proteins. In fact, the traditional view that membrane proteins may freely diffuse in a large 'sea of lipids' may need to be revised. Lastly, modifications of proteins by lipids or related derivatives have surprisingly complex roles on regulated intracellular transport of a wide range of molecules.
Figures
References
-
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. - PubMed
-
- Bankaitis VA, Morris AJ. Lipids and the exocytotic machinery of eukaryotic cells. Curr Opin Cell Biol. 2003;15:389–395. - PubMed
-
- van Meer G, Sprong H. Membrane lipids and vesicular traffic. Curr Opin Cell Biol. 2004;16:373–378. - PubMed
-
- Stace CL, Ktistakis NT. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta. 2006;1761:913–926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

