Costs and benefits of smoking cessation aids: making a case for public reimbursement of nicotine replacement therapy in Australia
- PMID: 17652241
- PMCID: PMC2598534
- DOI: 10.1136/tc.2006.017657
Costs and benefits of smoking cessation aids: making a case for public reimbursement of nicotine replacement therapy in Australia
Abstract
Background: Tobacco smoking is the leading preventable cause of morbidity and mortality in Australia and other developed countries. Of the pharmacological aids that are available for smoking cessation, bupropion (Zyban SR) is eligible for public reimbursement on the Australian Pharmaceutical Benefits Scheme (PBS), whereas nicotine replacement therapy (NRT) is not. Information on the cost-effectiveness and financial impact of public reimbursement of these strategies can better inform debate about their inclusion or exclusion in public reimbursement schemes.
Objective: To estimate the cost-effectiveness of bupropion and NRT, and the potential financial impact of public reimbursement of NRT in Australia.
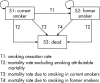
Design: A cost-effectiveness analysis using a deterministic Markov model, and cost per disability-adjusted life year (DALY) averted over a lifetime as the outcome measure.
Population: Current smokers, motivated to quit, in Australia in 2000.
Interventions: (1) NRT; (2) bupropion; and (3) a combined strategy using bupropion as the first-line treatment and NRT in those who fail to quit smoking or have adverse reactions to bupropion.
Results: Quitting smoking can increase life expectancy of current smokers by 1-7.6 years depending on age at cessation and sex. Providing bupropion to current smokers who are motivated to quit would cost A$7900 (95% uncertainty interval A$6000 to A$10,500) for each DALY averted; NRT patches would cost A$17,000 (A$9000 to A$28,000) for each DALY averted, with similar results even if used as a second-line treatment following initial failure to quit using bupropion. If 6% of current smokers were to use NRT following inclusion on the PBS, this would result in an annual cost of A$40-110 million to the PBS depending on the listed price.
Conclusions: Compared with other drugs included on the PBS, bupropion and NRT are both highly cost-effective smoking cessation interventions, and including NRT on the PBS would have a moderate financial impact. Given the sizeable health burden of smoking, and the large individual benefits of quitting smoking, increasing the availability of alternative aids and uptake of these strategies through public reimbursement would be a positive and rational step towards further reducing tobacco-related disease burden in Australia and other countries where NRT is currently not subsidised.
Conflict of interest statement
Competing interests: None declared.
References
-
- Vos T, Begg S.The Victorian Burden of Disease Study: mortality. Melbourne: Department of Human Services, 1999
-
- Silagy C, Lancaster T, Steadet al Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2002(4)CD000146 - PubMed
-
- World Health Organization Guidelines for controlling and monitoring the tobacco epidemic. Geneva: WHO, 1998
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials