Molecular determinants of antiviral potency of paramyxovirus entry inhibitors
- PMID: 17652384
- PMCID: PMC2045485
- DOI: 10.1128/JVI.01181-07
Molecular determinants of antiviral potency of paramyxovirus entry inhibitors
Abstract
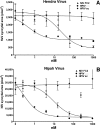
Hendra virus (HeV) and Nipah virus (NiV) constitute the Henipavirus genus of paramyxoviruses, both fatal in humans and with the potential for subversion as agents of bioterrorism. Binding of the HeV/NiV attachment protein (G) to its receptor triggers a series of conformational changes in the fusion protein (F), ultimately leading to formation of a postfusion six-helix bundle (6HB) structure and fusion of the viral and cellular membranes. The ectodomain of paramyxovirus F proteins contains two conserved heptad repeat regions, the first (the N-terminal heptad repeat [HRN]) adjacent to the fusion peptide and the second (the C-terminal heptad repeat [HRC]) immediately preceding the transmembrane domain. Peptides derived from the HRN and HRC regions of F are proposed to inhibit fusion by preventing activated F molecules from forming the 6HB structure that is required for fusion. We previously reported that a human parainfluenza virus 3 (HPIV3) F peptide effectively inhibits infection mediated by the HeV glycoproteins in pseudotyped-HeV entry assays more effectively than the comparable HeV-derived peptide, and we now show that this peptide inhibits live-HeV and -NiV infection. HPIV3 F peptides were also effective in inhibiting HeV pseudotype virus entry in a new assay that mimics multicycle replication. This anti-HeV/NiV efficacy can be correlated with the greater potential of the HPIV3 C peptide to interact with the HeV F N peptide coiled-coil trimer, as evaluated by thermal unfolding experiments. Furthermore, replacement of a buried glutamic acid (glutamic acid 459) in the C peptide with valine enhances antiviral potency and stabilizes the 6HB conformation. Our results strongly suggest that conserved interhelical packing interactions in the F protein fusion core are important determinants of C peptide inhibitory activity and offer a strategy for the development of more-potent analogs of F peptide inhibitors.
Figures




References
-
- Aguilar, H. C., K. A. Matreyek, C. M. Filone, S. T. Hashimi, E. L. Levroney, O. A. Negrete, A. Bertolotti-Ciarlet, D. Y. Choi, I. McHardy, J. A. Fulcher, S. V. Su, M. C. Wolf, L. Kohatsu, L. G. Baum, and B. Lee. 2006. N-glycans on Nipah virus fusion protein protect against neutralization but reduce membrane fusion and viral entry. J. Virol. 80:4878-4889. - PMC - PubMed
-
- Anfinsen, C. B. 1973. Principles that govern the folding of protein chains. Science 181:223-230. - PubMed
-
- Arakawa, T., and S. N. Timasheff. 1984. Protein stabilization and destabilization by guanidinium salts. Biochemistry 23:5924-5929. - PubMed
-
- Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

