Effects of type I interferons on the adjuvant properties of plasmid granulocyte-macrophage colony-stimulating factor in vivo
- PMID: 17652387
- PMCID: PMC2045443
- DOI: 10.1128/JVI.01000-07
Effects of type I interferons on the adjuvant properties of plasmid granulocyte-macrophage colony-stimulating factor in vivo
Abstract
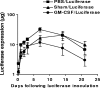
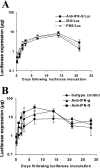
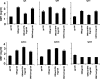
While administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) can induce the local recruitment of activated antigen-presenting cells at the site of vaccine inoculation, this cellular recruitment is associated with a paradoxical decrease in local vaccine antigen expression and vaccine-elicited CD8+ T-cell responses. To clarify why this cytokine administration does not potentiate immunization, we examined the recruited cells and expressed inflammatory mediators in muscles following intramuscular administration of plasmid GM-CSF in mice. While large numbers of dendritic cells and macrophages were attracted to the site of plasmid GM-CSF inoculation, high concentrations of type I interferons were also detected in the muscles. As type I interferons have been reported to damp foreign gene expression in vivo, we examined the possibility that these local innate mediators might decrease plasmid DNA expression and therefore the immunogenicity of plasmid DNA vaccines. In fact, we found that coadministration of an anti-beta interferon monoclonal antibody with the plasmid DNA immunogen and plasmid GM-CSF restored both the local antigen expression and the CD8+ T-cell immunogenicity of the vaccine. These data demonstrate that local innate immune responses can change the ability of vaccines to generate robust adaptive immunity.
Figures

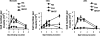


Similar articles
-
Immune-enhancing effect of nano-DNA vaccine encoding a gene of the prME protein of Japanese encephalitis virus and BALB/c mouse granulocyte-macrophage colony-stimulating factor.Mol Med Rep. 2015 Jul;12(1):199-209. doi: 10.3892/mmr.2015.3419. Epub 2015 Mar 4. Mol Med Rep. 2015. PMID: 25738258 Free PMC article.
-
Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles.J Immunol. 2000 Oct 1;165(7):3772-81. doi: 10.4049/jimmunol.165.7.3772. J Immunol. 2000. PMID: 11034382
-
Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF.J Immunol. 2002 Jan 15;168(2):562-8. doi: 10.4049/jimmunol.168.2.562. J Immunol. 2002. PMID: 11777947
-
Modulation of immune responses to DNA vaccines by codelivery of cytokine genes.J Formos Med Assoc. 1999 Nov;98(11):722-9. J Formos Med Assoc. 1999. PMID: 10705687 Review.
-
Uses of granulocyte-macrophage colony-stimulating factor in vaccine development.Curr Opin Hematol. 2000 May;7(3):168-73. doi: 10.1097/00062752-200005000-00007. Curr Opin Hematol. 2000. PMID: 10786654 Review.
Cited by
-
Differential Type I Interferon Signaling Is a Master Regulator of Susceptibility to Postinfluenza Bacterial Superinfection.mBio. 2016 May 3;7(3):e00506-16. doi: 10.1128/mBio.00506-16. mBio. 2016. PMID: 27143388 Free PMC article.
-
Virus Eradication and Synthetic Biology: Changes with SARS-CoV-2?Viruses. 2021 Mar 28;13(4):569. doi: 10.3390/v13040569. Viruses. 2021. PMID: 33800626 Free PMC article.
-
A simultaneous oral and intramuscular prime/sublingual boost with a DNA/Modified Vaccinia Ankara viral vector-based vaccine induces simian immunodeficiency virus-specific systemic and mucosal immune responses in juvenile rhesus macaques.J Med Primatol. 2018 Oct;47(5):288-297. doi: 10.1111/jmp.12372. Epub 2018 Sep 11. J Med Primatol. 2018. PMID: 30204253 Free PMC article.
-
Regulatory T Cells Modulate DNA Vaccine Immunogenicity at Early Time via Functional CD4(+) T Cells and Antigen Duration.Front Immunol. 2015 Sep 29;6:510. doi: 10.3389/fimmu.2015.00510. eCollection 2015. Front Immunol. 2015. PMID: 26483796 Free PMC article.
-
Oral Coadministration of an Intramuscular DNA/Modified Vaccinia Ankara Vaccine for Simian Immunodeficiency Virus Is Associated with Better Control of Infection in Orally Exposed Infant Macaques.AIDS Res Hum Retroviruses. 2019 Mar;35(3):310-325. doi: 10.1089/AID.2018.0180. Epub 2018 Nov 27. AIDS Res Hum Retroviruses. 2019. PMID: 30303405 Free PMC article.
References
-
- Arpinati, M., C. L. Green, S. Heimfeld, J. E. Heuser, and C. Anasetti. 2000. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95:2484-2490. - PubMed
-
- Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. - PubMed
-
- Bauer, M., V. Redecke, J. W. Ellwart, B. Scherer, J. P. Kremer, H. Wagner, and G. B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000-5007. - PubMed
-
- Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. - PubMed
-
- Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

