Association of the astrovirus structural protein VP90 with membranes plays a role in virus morphogenesis
- PMID: 17652389
- PMCID: PMC2045458
- DOI: 10.1128/JVI.00785-07
Association of the astrovirus structural protein VP90 with membranes plays a role in virus morphogenesis
Abstract
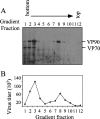
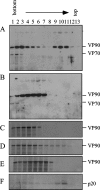
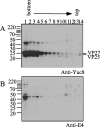
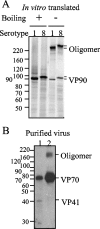
VP90, the capsid polyprotein precursor of human astrovirus Yuc8, is assembled into viral particles, and its processing at the carboxy terminus by cellular caspases, to yield VP70, has been correlated with the cell release of the virus. Here, we characterized the effect of the VP90-VP70 processing on the properties of these proteins, as well as on their intracellular distribution. VP90 was found in membrane-enriched fractions (mVP90), as well as in fractions enriched in cytosolic proteins (cVP90), while VP70 was found exclusively in the latter fractions. Upon trypsin activation, infectivity was detected in all VP90-containing fractions, confirming that both mVP90 and cVP90 are able to assemble into particles; however, the two forms of VP90 showed differential sensitivities to trypsin, especially at their carboxy termini, which in the case of mVP90 was shown to remain membrane associated after protease digestion. Structural protein oligomers were detected in purified VP70-containing viruses, as well as in membrane-enriched fractions, but they were less evident in cytosolic fractions. Ultrastructural studies of infected cells revealed different types of viral particles, some of which appeared to be associated with membranes. By immunoelectron microscopy, structural proteins were shown to form virus particles in clusters and to associate with the edges of vesicles induced during infection, which also appear to contain subviral particles inside. Nonstructural proteins and viral RNA colocalized with mVP90, but not with cVP90, suggesting that mVP90 might represent the form of the protein that is initially assembled into particles, at the sites where the virus genome is being replicated.
Figures
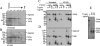




References
-
- Belliot, G., H. Laveran, and S. S. Monroe. 1997. Capsid protein composition of reference strains and wild isolates of human astroviruses. Virus Res. 49:49-57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

