Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium
- PMID: 17652457
- PMCID: PMC1995711
- DOI: 10.1091/mbc.e07-03-0205
Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium
Abstract
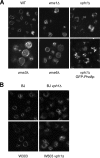
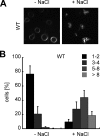
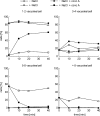
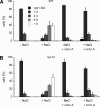
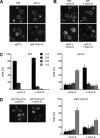
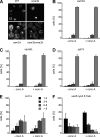
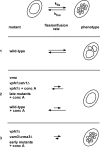
Like numerous other eukaryotic organelles, the vacuole of the yeast Saccharomyces cerevisiae undergoes coordinated cycles of membrane fission and fusion in the course of the cell cycle and in adaptation to environmental conditions. Organelle fission and fusion processes must be balanced to ensure organelle integrity. Coordination of vacuole fission and fusion depends on the interactions of vacuolar SNARE proteins and the dynamin-like GTPase Vps1p. Here, we identify a novel factor that impinges on the fusion-fission equilibrium: the vacuolar H(+)-ATPase (V-ATPase) performs two distinct roles in vacuole fission and fusion. Fusion requires the physical presence of the membrane sector of the vacuolar H(+)-ATPase sector, but not its pump activity. Vacuole fission, in contrast, depends on proton translocation by the V-ATPase. Eliminating proton pumping by the V-ATPase either pharmacologically or by conditional or constitutive V-ATPase mutations blocked salt-induced vacuole fragmentation in vivo. In living cells, fission defects are epistatic to fusion defects. Therefore, mutants lacking the V-ATPase display large single vacuoles instead of multiple smaller vacuoles, the phenotype that is generally seen in mutants having defects only in vacuolar fusion. Its dual involvement in vacuole fission and fusion suggests the V-ATPase as a potential regulator of vacuolar morphology and membrane dynamics.
Figures
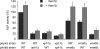

References
-
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., Hieter P., Boeke J. D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. - PubMed
-
- Chan D. C. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

