The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin
- PMID: 17652459
- PMCID: PMC1995720
- DOI: 10.1091/mbc.e07-01-0075
The Abl-related gene tyrosine kinase acts through p190RhoGAP to inhibit actomyosin contractility and regulate focal adhesion dynamics upon adhesion to fibronectin
Abstract
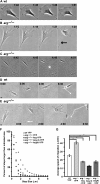
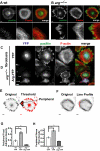
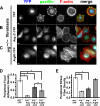
In migrating cells, actin polymerization promotes protrusion of the leading edge, whereas actomyosin contractility powers net cell body translocation. Although they promote F-actin-dependent protrusions of the cell periphery upon adhesion to fibronectin (FN), Abl family kinases inhibit cell migration on FN. We provide evidence here that the Abl-related gene (Arg/Abl2) kinase inhibits fibroblast migration by attenuating actomyosin contractility and regulating focal adhesion dynamics. arg-/- fibroblasts migrate at faster average speeds than wild-type (wt) cells, whereas Arg re-expression in these cells slows migration. Surprisingly, the faster migrating arg-/- fibroblasts have more prominent F-actin stress fibers and focal adhesions and exhibit increased actomyosin contractility relative to wt cells. Interestingly, Arg requires distinct functional domains to inhibit focal adhesions and actomyosin contractility. The kinase domain-containing Arg N-terminal half can act through the RhoA inhibitor p190RhoGAP to attenuate stress fiber formation and cell contractility. However, Arg requires both its kinase activity and its cytoskeleton-binding C-terminal half to fully inhibit focal adhesions. Although focal adhesions do not turn over efficiently in the trailing edge of arg-/- cells, the increased contractility of arg-/- cells tears the adhesions from the substrate, allowing for the faster migration observed in these cells. Together, our data strongly suggest that Arg inhibits cell migration by restricting actomyosin contractility and regulating its coupling to the substrate through focal adhesions.
Figures



References
-
- Affolter M., Weijer C. J. Signaling to cytoskeletal dynamics during chemotaxis. Dev. Cell. 2005;9:19–34. - PubMed
-
- Arora P. D., McCulloch C. A. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J. Cell. Physiol. 1994;159:161–175. - PubMed
-
- Bashaw G. J., Kidd T., Murray D., Pawson T., Goodman C. S. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. - PubMed
-
- Bennett R. L., Hoffmann F. M. Increased levels of the Drosophila Abelson tyrosine kinase in nerves and muscles: subcellular localization and mutant phenotypes imply a role in cell-cell interactions. Development. 1992;116:953–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

