Primary sensory neuron addition in the adult rat trigeminal ganglion: evidence for neural crest glio-neuronal precursor maturation
- PMID: 17652585
- PMCID: PMC6672737
- DOI: 10.1523/JNEUROSCI.1203-07.2007
Primary sensory neuron addition in the adult rat trigeminal ganglion: evidence for neural crest glio-neuronal precursor maturation
Abstract
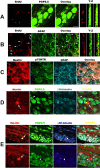
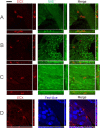
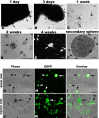
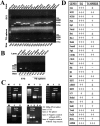
It is debated whether primary sensory neurons of the dorsal root ganglia increase the number in adult animals and, if so, whether the increase is attributable to postnatal neurogenesis or maturation of dormant, postmitotic precursors. Similar studies are lacking in the trigeminal ganglion (TG). Here we demonstrate by stereological methods that the number of neurons in the TG of adult male rats nearly doubles between the third and eighth months of age. The increase is mainly attributable to the addition of small, B-type neurons, with a smaller contribution of large, A-neurons. We looked for possible proliferative or maturation mechanisms that could explain this dramatic postnatal expansion in neuron number, using bromodeoxyuridine (BrdU) labeling, immunocytochemistry for neural precursor cell antigens, retrograde tracing identification of peripherally projecting neurons, and in vitro isolation of precursor cells from adult TG explant cultures. Cell proliferation identified months after an extended BrdU administration was sparse and essentially corresponded to glial cells. No BrdU-labeled cell took up the peripherally injected tracer, and only a negligible number coexpressed BrdU and the pan-neuronal tracer neuron-specific enolase. In contrast, a population of cells not recognizable as mature neurons in the TG and neighboring nerve expressed neuronal precursor antigens, and neural crest glioneuronal precursor cells were successfully isolated from adult TG explants. Our data suggest that a protracted maturation process persists in the TG that can be responsible for the neuronal addition found in the adult rat.
Figures





Similar articles
-
Identification of dividing, determined sensory neuron precursors in the mammalian neural crest.Development. 1999 Aug;126(16):3545-59. doi: 10.1242/dev.126.16.3545. Development. 1999. PMID: 10409501
-
Pax3-expressing trigeminal placode cells can localize to trunk neural crest sites but are committed to a cutaneous sensory neuron fate.Dev Biol. 2002 Sep 15;249(2):219-36. doi: 10.1006/dbio.2002.0767. Dev Biol. 2002. PMID: 12221003
-
Dividing neuron precursors express neuron-specific tubulin.J Neurobiol. 1995 May;27(1):26-43. doi: 10.1002/neu.480270104. J Neurobiol. 1995. PMID: 7643073
-
Specification of sensory neuron cell fate from the neural crest.Adv Exp Med Biol. 2006;589:170-80. doi: 10.1007/978-0-387-46954-6_10. Adv Exp Med Biol. 2006. PMID: 17076281 Review.
-
Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia.Brain Res Bull. 2002 Apr;57(6):809-16. doi: 10.1016/s0361-9230(01)00767-5. Brain Res Bull. 2002. PMID: 12031277 Review.
Cited by
-
Generation of new neurons in dorsal root Ganglia in adult rats after peripheral nerve crush injury.Neural Plast. 2015;2015:860546. doi: 10.1155/2015/860546. Epub 2015 Feb 3. Neural Plast. 2015. PMID: 25722894 Free PMC article.
-
Molecular Segmentation of the Spinal Trigeminal Nucleus in the Adult Mouse Brain.Front Neuroanat. 2021 Dec 10;15:785840. doi: 10.3389/fnana.2021.785840. eCollection 2021. Front Neuroanat. 2021. PMID: 34955765 Free PMC article.
-
5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice.J Neurosci. 2009 Aug 5;29(31):9683-99. doi: 10.1523/JNEUROSCI.1145-09.2009. J Neurosci. 2009. PMID: 19657021 Free PMC article.
-
Basic fibroblast growth factor (bFGF) facilitates differentiation of adult dorsal root ganglia-derived neural stem cells toward Schwann cells by binding to FGFR-1 through MAPK/ERK activation.J Mol Neurosci. 2014 Apr;52(4):538-51. doi: 10.1007/s12031-013-0109-2. Epub 2013 Sep 27. J Mol Neurosci. 2014. PMID: 24072480
-
Postembryonic neuronal addition in zebrafish dorsal root ganglia is regulated by Notch signaling.Neural Dev. 2012 Jun 27;7:23. doi: 10.1186/1749-8104-7-23. Neural Dev. 2012. PMID: 22738203 Free PMC article.
References
-
- Aigner L, Uyanik G, Couillard-Despres S, Ploetz S, Wolff G, Morris-Rosendahl D, Martin P, Eckel U, Spranger S, Otte J, Woerle H, Holthausen H, Apheshiotis N, Fluegel D, Winkler J. Somatic mosaicism and variable penetrance in doublecortin-associated migration disorders. Neurology. 2003;60:329–332. - PubMed
-
- Aldskogius H, Arvidsson J. Nerve cell degeneration and death in the trigeminal ganglion of the adult rat following peripheral nerve transection. J Neurocytol. 1978;7:229–250. - PubMed
-
- Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. - PubMed
-
- Anderson DJ. Lineages and transcription factors in the specification of vertebrate primary sensory neurons. Curr Opin Neurobiol. 1999;9:517–524. - PubMed
-
- Andres KH. Untersuchungen den Feinbau von spinal Ganglien. Z Zellforsch Mikr Anat. 1961;55:1–48. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
