Behavioral and in vitro correlates of compulsive-like food seeking induced by operant conditioning in Aplysia
- PMID: 17652597
- PMCID: PMC6672725
- DOI: 10.1523/JNEUROSCI.1950-07.2007
Behavioral and in vitro correlates of compulsive-like food seeking induced by operant conditioning in Aplysia
Abstract
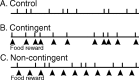
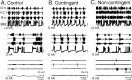
Motivated behaviors comprise appetitive actions whose occurrence results partly from an internally driven incentive to act. Such impulsive behavior can also be regulated by external rewarding stimuli that, through learning processes, can lead to accelerated and seemingly automatic, compulsive-like recurrences of the rewarded act. Here, we explored such behavioral plasticity in Aplysia by analyzing how appetitive reward stimulation in a form of operant conditioning can modify a goal-directed component of the animal's food-seeking behavior. In naive animals, protraction/retraction cycles of the tongue-like radula are expressed sporadically with highly variable interbite intervals. In contrast, animals that were previously given a food-reward stimulus in association with each spontaneous radula bite now expressed movement cycles with an elevated frequency and a stereotyped rhythmic organization. This rate increase and regularization, which was retained for several hours after training, depended on both the reward quality and its contingency because accelerated, stereotyped biting was not induced in animals that had previously received a less-palatable food stimulus or had been subjected to nonassociative reward stimulation. Neuronal correlates of these learning-induced changes were also expressed in the radula motor pattern-generating circuitry of isolated buccal ganglia. In such in vitro preparations, moreover, manipulation of the burst frequency of the bilateral motor pattern-initiating B63 interneurons indicated that the regularization of radula motor pattern generation in contingently trained animals occurred separately from an increase in cycle rate, thereby suggesting independent processes of network plasticity. These data therefore suggest that operant conditioning can induce compulsive-like actions in Aplysia feeding behavior and provide a substrate for a cellular analysis of the underlying mechanisms.
Figures








References
-
- Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. - PubMed
-
- Belozertseva IV, Sukhotina IA, Vossen JMH, Bespalov AY. Facilitation of aggressive and sexual behaviors by saccharin deprivation in rats. Physiol Behav. 2004;80:531–539. - PubMed
-
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH. Operant reward learning in Aplysia: neuronal correlates and mechanisms. Science. 2002;296:1706–1709. - PubMed
-
- Canavier CC, Baxter DA, Clark JW, Byrne JH. Multiple modes of activity in a model neuron suggest a novel mechanism for the effects of neuromodulators. J Neurophysiol. 1994;72:872–882. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
