A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon
- PMID: 17652602
- PMCID: PMC6672716
- DOI: 10.1523/JNEUROSCI.1420-07.2007
A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon
Abstract
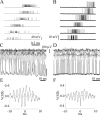
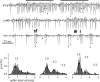
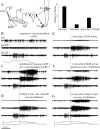
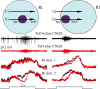
We describe the operation of a midbrain neural circuit in pigeons that may participate in selecting and attending to one visual stimulus from the myriad displayed in their visual environment. This mechanism is based on a topographically organized cholinergic signal reentering the optic tectum (TeO). We have shown previously that, whenever a visual stimulus activates neurons in a given tectal location, this location receives a strong bursting feedback from cholinergic neurons of the nucleus isthmi pars parvocellularis (Ipc), situated underneath the tectum. Here we show that, if a second visual stimulus is presented, even far from the first, the feedback signal to the first tectal location is diminished or suppressed, and feedback to the second tectal location is initiated. We found that this long-range suppressive interaction is mostly mediated by the nucleus isthmi pars magnocellularis, which sends a wide-field GABAergic projection to Ipc and TeO. In addition, two sets of findings indicate that the feedback from the Ipc modulates the ascending output from the TeO. First, visually evoked extracellular responses recorded in the dorsal anterior subdivision of the thalamic nucleus rotundus (RtDa), receiving the ascending tectal output, are closely synchronized to this feedback signal. Second, local inactivation of the Ipc prevents visual responses in RtDa to visual targets moving in the corresponding region of visual space. These results suggest that the ascending transmission of visual activity through the tectofugal pathway is gated by this cholinergic re-entrant signal, whose location within the tectal visual map is dynamically defined by competitive interactions.
Figures




References
-
- Appell PP, Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990;302:143–158. - PubMed
-
- Binns KE, Salt TE. The functional influence of nicotinic cholinergic receptors on the visual responses of neurones in the superficial superior colliculus. Vis Neurosci. 2000;17:283–289. - PubMed
-
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
