Integrating S-phase checkpoint signaling with trans-lesion synthesis of bulky DNA adducts
- PMID: 17652783
- PMCID: PMC3103048
- DOI: 10.1007/s12013-007-0032-7
Integrating S-phase checkpoint signaling with trans-lesion synthesis of bulky DNA adducts
Abstract
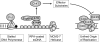
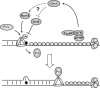
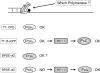
Bulky adducts are DNA lesions generated in response to environmental agents including benzo[a]pyrene (a combustion product) and solar ultraviolet radiation. Error-prone replication of adducted DNA can cause mutations, which may result in cancer. To minimize the detrimental effects of bulky adducts and other DNA lesions, S-phase checkpoint mechanisms sense DNA damage and integrate DNA repair with ongoing DNA replication. The essential protein kinase Chk1 mediates the S-phase checkpoint, inhibiting initiation of new DNA synthesis and promoting stabilization and recovery of stalled replication forks. Here we review the mechanisms by which Chk1 is activated in response to bulky adducts and potential mechanisms by which Chk1 signaling inhibits the initiation stage of DNA synthesis. Additionally, we discuss mechanisms by which Chk1 signaling facilitates bypass of bulky lesions by specialized Y-family DNA polymerases, thereby attenuating checkpoint signaling and allowing resumption of normal cell cycle progression.
Figures
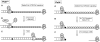
Similar articles
-
The DNA damage response kinases DNA-dependent protein kinase (DNA-PK) and ataxia telangiectasia mutated (ATM) Are stimulated by bulky adduct-containing DNA.J Biol Chem. 2011 Jun 3;286(22):19237-46. doi: 10.1074/jbc.M111.235036. Epub 2011 Apr 12. J Biol Chem. 2011. PMID: 21487018 Free PMC article.
-
Multiple ATR-Chk1 pathway proteins preferentially associate with checkpoint-inducing DNA substrates.PLoS One. 2011;6(7):e22986. doi: 10.1371/journal.pone.0022986. Epub 2011 Jul 29. PLoS One. 2011. PMID: 21829571 Free PMC article.
-
DNA adducts from a tumorigenic metabolite of benzo[a]pyrene block human RNA polymerase II elongation in a sequence- and stereochemistry-dependent manner.J Mol Biol. 2002 Aug 2;321(1):29-47. doi: 10.1016/s0022-2836(02)00593-4. J Mol Biol. 2002. PMID: 12139931
-
Regulation of ATR-CHK1 signaling by ubiquitination of CLASPIN.Biochem Soc Trans. 2022 Oct 31;50(5):1471-1480. doi: 10.1042/BST20220729. Biochem Soc Trans. 2022. PMID: 36196914 Review.
-
Replication checkpoint: preventing mitotic catastrophe.Curr Biol. 2001 Feb 20;11(4):R121-4. doi: 10.1016/s0960-9822(01)00057-4. Curr Biol. 2001. PMID: 11250164 Review.
Cited by
-
c-Jun N-terminal kinase-mediated Rad18 phosphorylation facilitates Polη recruitment to stalled replication forks.Mol Biol Cell. 2012 May;23(10):1943-54. doi: 10.1091/mbc.E11-10-0829. Epub 2012 Mar 28. Mol Biol Cell. 2012. PMID: 22456510 Free PMC article.
-
Antitumor Activity, Mechanisms of Action and Phytochemical Profiling of Sub-Fractions Obtained from Ulex gallii Planch. (Fabaceae): A Medicinal Plant from Galicia (Spain).Molecules. 2025 Feb 19;30(4):972. doi: 10.3390/molecules30040972. Molecules. 2025. PMID: 40005281 Free PMC article.
-
Identification of a novel REV1-interacting motif necessary for DNA polymerase kappa function.Genes Cells. 2009 Feb;14(2):101-11. doi: 10.1111/j.1365-2443.2008.01255.x. Epub 2009 Jan 6. Genes Cells. 2009. PMID: 19170759 Free PMC article.
-
Folate-based binuclear Mn(II) chelates with 2,2'-bipyridine/1,10-phenanthroline as targeted anticancer agents for colon cancer cells.Sci Rep. 2025 Jul 31;15(1):27905. doi: 10.1038/s41598-025-12251-9. Sci Rep. 2025. PMID: 40744967 Free PMC article.
-
Global regulation of genome duplication in eukaryotes: an overview from the epifluorescence microscope.Chromosoma. 2008 Jun;117(3):243-60. doi: 10.1007/s00412-007-0145-1. Epub 2008 Jan 16. Chromosoma. 2008. PMID: 18197411 Review.
References
-
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. - PubMed
-
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. - PubMed
-
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. - PubMed
-
- Baum EJ. Occurrence and surveillance of polycyclic aromatic hydrocarbons. Academic Press; NY: 1978.
-
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

