A randomized trial evaluating Prosaptide for HIV-associated sensory neuropathies: use of an electronic diary to record neuropathic pain
- PMID: 17653259
- PMCID: PMC1919427
- DOI: 10.1371/journal.pone.0000551
A randomized trial evaluating Prosaptide for HIV-associated sensory neuropathies: use of an electronic diary to record neuropathic pain
Abstract
Objectives: To examine the efficacy and safety of Prosaptide (PRO) for the treatment of painful HIV-associated sensory neuropathies (HIV-SN).
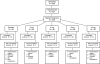
Design: A randomized, double-blind, placebo-controlled, multicenter study in participants with sensory neuropathy. Pain modulating therapy was discontinued prior to baseline. Participants were stratified by sural sensory nerve action potential (SNAP) amplitude. Participants were trained to use an electronic diary (ED) to record pain.
Setting: Peripheral neuropathies are common complications of HIV infection. The pathogenesis is unknown and currently treatments are restricted to symptomatic measures. We examined PRO against placebo (PBO) for treatment of painful HIV-SN and performed a post-hoc evaluation of an electronic diary (ED) to record HIV-associated neuropathic pain.
Participants: Eligible participants included adults with neurologist-confirmed painful HIV-SN.
Interventions: 2, 4, 8, or 16 mg/d PRO or PBO administered via subcutaneous (SC) injection for six weeks. Neurotoxic antiretroviral drug usage was held constant.
Outcome measures: Changes from baseline in the weekly average of evaluable daily random prompts measuring pain using the Gracely pain scale and adverse events.
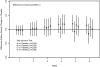
Results: 237 participants were randomized. The study was stopped after a planned futility analysis. There were no between-group differences in the frequency of adverse events or laboratory toxicities. The 6-week mean (sd) Gracely pain scale changes were -0.12 (0.23), -0.24 (0.35), -0.15 (0.32), -0.18 (0.34), and -0.18 (0.32) for the 2, 4, 8, 16 mg, and PBO arms respectively. A similar variability of pain changes recorded using the ED were noted compared to previous trials that used paper collection methods.
Conclusions: 6-week treatment with PRO was safe but not effective at reducing HIV-associated neuropathic pain. Use of an ED to record neuropathic pain is novel in HIV-SN, resulted in reasonable compliance in recording pain data, but did not decrease the variability of pain scores compared to historical paper collection methods.
Trial registration: Current Controlled Trials NCT00286377.
Conflict of interest statement
Figures
References
-
- Sacktor N, Lyles R, Skolasky R, Kleeberger C, Selnes OA, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study. Neurology. 2001;56:257–260. - PubMed
-
- Bacellar H, Munoz A, Miller EN, Cohen BA, Besley D, et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study, 1985–1992. Neurology. 1984;44:1892–1900. - PubMed
-
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurology. 2005;4(9):543–555. - PubMed
-
- Wulff E, Wang A, Simpson D. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. - PubMed
-
- Cornblath D, McArthur J. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;1938:794–796. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P30 MH075673/MH/NIMH NIH HHS/United States
- P20 RR011126/RR/NCRR NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- R01 NS044807/NS/NINDS NIH HHS/United States
- U01 AI027668/AI/NIAID NIH HHS/United States
- U01 AI027670/AI/NIAID NIH HHS/United States
- U54 NS043011/NS/NINDS NIH HHS/United States
- U01 AI025903/AI/NIAID NIH HHS/United States
- U01 AI027660/AI/NIAID NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- U01 AI046386/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- U01 AI046376/AI/NIAID NIH HHS/United States
- U01 NS032228/NS/NINDS NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- K24 NS002253/NS/NINDS NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- U01 AI027658/AI/NIAID NIH HHS/United States
- U01 AI027675/AI/NIAID NIH HHS/United States
- R01 NS032228/NS/NINDS NIH HHS/United States
- R01 NS049465/NS/NINDS NIH HHS/United States
- U01 AI032783/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical