Simple PCR assays improve the sensitivity of HIV-1 subtype B drug resistance testing and allow linking of resistance mutations
- PMID: 17653265
- PMCID: PMC1919426
- DOI: 10.1371/journal.pone.0000638
Simple PCR assays improve the sensitivity of HIV-1 subtype B drug resistance testing and allow linking of resistance mutations
Abstract
Background: The success of antiretroviral therapy is known to be compromised by drug-resistant HIV-1 at frequencies detectable by conventional bulk sequencing. Currently, there is a need to assess the clinical consequences of low-frequency drug resistant variants occurring below the detection limit of conventional genotyping. Sensitive detection of drug-resistant subpopulations, however, requires simple and practical methods for routine testing.
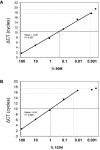
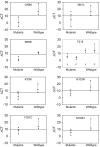
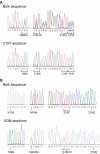
Methodology: We developed highly-sensitive and simple real-time PCR assays for nine key drug resistance mutations and show that these tests overcome substantial sequence heterogeneity in HIV-1 clinical specimens. We specifically used early wildtype virus samples from the pre-antiretroviral drug era to measure background reactivity and were able to define highly-specific screening cut-offs that are up to 67-fold more sensitive than conventional genotyping. We also demonstrate that sequencing the mutation-specific PCR products provided a direct and novel strategy to further detect and link associated resistance mutations, allowing easy identification of multi-drug-resistant variants. Resistance mutation associations revealed in mutation-specific amplicon sequences were verified by clonal sequencing.
Significance: Combined, sensitive real-time PCR testing and mutation-specific amplicon sequencing provides a powerful and simple approach that allows for improved detection and evaluation of HIV-1 drug resistance mutations.
Conflict of interest statement
Figures
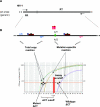
References
-
- US Department of Health and Human Services. 2006. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. . Available: http://AIDSinfo.nih.gov.
-
- Johnson JA, Li J-F, Morris L, Martinson N, Gray G, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192:16–23. - PubMed
-
- Flys T, Nissley DV, Claasen CW, Jones D, Shi C, et al. Sensitive drug resistance assays reveal long-term persistence of HIV-1 variants with the K103N nevirapine (NVP) resistance mutation in some women and infants after administration of single-dose NVP: HIVNET 012. J Infect Dis. 2005;192:24–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

