Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology
- PMID: 17653280
- PMCID: PMC1919432
- DOI: 10.1371/journal.pone.0000653
Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology
Abstract
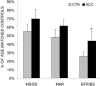
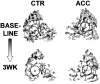
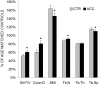
A range of tissues have the capacity to adapt to mechanical challenges, an attribute presumed to be regulated through deformation of the cell and/or surrounding matrix. In contrast, it is shown here that extremely small oscillatory accelerations, applied as unconstrained motion and inducing negligible deformation, serve as an anabolic stimulus to osteoblasts in vivo. Habitual background loading was removed from the tibiae of 18 female adult mice by hindlimb-unloading. For 20 min/d, 5 d/wk, the left tibia of each mouse was subjected to oscillatory 0.6 g accelerations at 45 Hz while the right tibia served as control. Sham-loaded (n = 9) and normal age-matched control (n = 18) mice provided additional comparisons. Oscillatory accelerations, applied in the absence of weight bearing, resulted in 70% greater bone formation rates in the trabeculae of the metaphysis, but similar levels of bone resorption, when compared to contralateral controls. Quantity and quality of trabecular bone also improved as a result of the acceleration stimulus, as evidenced by a significantly greater bone volume fraction (17%) and connectivity density (33%), and significantly smaller trabecular spacing (-6%) and structural model index (-11%). These in vivo data indicate that mechanosensory elements of resident bone cell populations can perceive and respond to acceleratory signals, and point to an efficient means of introducing intense physical signals into a biologic system without putting the matrix at risk of overloading. In retrospect, acceleration, as opposed to direct mechanical distortion, represents a more generic and safe, and perhaps more fundamental means of transducing physical challenges to the cells and tissues of an organism.
Conflict of interest statement
Figures

References
-
- Galileo G. Discorsi e dimonstrazioni matematiche, intorno a due nuove scienze attentanti alla meccanica ed a muovementi localli. Madison, WI: University of Wisconsin Press; 1638.
-
- Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. - PubMed
-
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. - PubMed
-
- Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. - PubMed
-
- Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism: Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

