Commensal bacteria and expression of two major intestinal chemokines, TECK/CCL25 and MEC/CCL28, and their receptors
- PMID: 17653288
- PMCID: PMC1919421
- DOI: 10.1371/journal.pone.0000677
Commensal bacteria and expression of two major intestinal chemokines, TECK/CCL25 and MEC/CCL28, and their receptors
Abstract
Background: CCL25/TECK and CCL28/MEC are CC chemokines primarily expressed in thymic dendritic cells and mucosal epithelial cells. Their receptors, CCR9 and CCR10, are mainly expressed on T and B lymphocytes. In human, mouse, pig and sheep CCL25 and CCL28 play an important role in the segregation and the compartmentalization of the mucosal immune system. As evidenced by early comparisons of germ-free and conventional animals, the intestinal bacterial microflora has a marked effect on host intestinal immune functions. However, little is known about the impact of bacterial colonization on constitutive and induced chemokine expressions as well as on the generation of anti-inflammatory mechanisms.
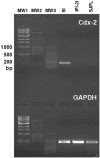
Methodology/principal findings: Therefore, we decided to focus by qPCR on the mRNA expression of two main gut chemokines, CCL25 and CCL28, their receptors CCR9 and CCR10, the Tregs marker Foxp3 and anti-inflammatory cytokines TGF-beta and IL-10 following colonization with different bacterial species within the small intestine. To accomplish this we used an original germ-free neonatal pig model and monoassociated pigs with a representative Gram-negative (Escherichia coli) or Gram-positive (Lactobacillus fermentum) commensal bacteria commonly isolated from the neonatal pig intestine. Our results show a consistent and marked effect of microbial colonization on the mRNA expression of intestinal chemokines, chemokine receptors, Foxp3 and TGF-beta. Moreover, as evidenced by in vitro experiments using two different cell lines, the pattern of regulation of CCL25 and CCL28 expression in the gut appears complex and suggests an additional role for in vivo factors.
Conclusions/significance: Taken together, the results highlight the key role of bacterial microflora in the development of a functional intestinal immune system in an elegant and relevant model for human immune system development.
Conflict of interest statement
Figures






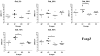
References
-
- Pastoret P-P, Griebel P, Bazin H, Govaerts A. Immunology of the pig. In: Pastoret P-P, Griebel P, Bazin H, Govaerts A, editors. Handbook of vertebrate immunology. California, USA: Academic Press; 1998. pp. 373–419.
-
- Tumbleson M, Schook LB. Advances in swine in biomedical research. In: Tumbleson M, Schook LB, editors. Advances in swine in biomedical research. New-York, USA: Plenum Press; 1996. pp. 1–4.
-
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. - PubMed
-
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. - PubMed
-
- Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

