Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation
- PMID: 17654295
- PMCID: PMC2039873
- DOI: 10.1080/14622200701382033
Pharmacogenetic clinical trial of sustained-release bupropion for smoking cessation
Erratum in
- Nicotine Tob Res. 2007 Nov;9(11):1243
Abstract
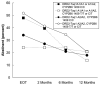
This randomized, double-blinded, placebo-controlled trial examined genetic influences on treatment response to sustained-release bupropion for smoking cessation. Smokers of European ancestry (N = 291), who were randomized to receive bupropion or placebo (12 weeks) plus counseling, were genotyped for the dopamine D2 receptor (DRD2-Taq1A), dopamine transporter (SLC6A3 3' VNTR), and cytochrome P450 2B6 (CYP2B6 1459 CT) polymorphisms. Main outcome measures were cotinine-verified point prevalence of abstinence at end of treatment and at 2-, 6-, and 12-month follow-ups post quit date. Using generalized estimating equations, we found that bupropion, compared with placebo, was associated with significantly greater odds of abstinence at all time points (all p values<.01). We found a significant DRD2 x bupropion interaction (B = 1.49, SE = 0.59, p = .012) [corrected] and a three-way DRD2 x bupropion x craving interaction on 6-month smoking cessation outcomes (B = -0.45, SE = 0.22, p = .038), such that smokers with the A2/A2 genotype demonstrated the greatest craving reduction and the highest abstinence rates with bupropion. Furthermore, there was a significant DRD2 x CYP2B6 interaction (B = 1.43, SE = 0.56, p = .01), such that individuals with the DRD2-Taq1 A2/A2 genotype demonstrated a higher odds of abstinence only if they possessed the CYP2B6 1459 T/T or C/T genotype. Because the sample size of this study was modest for pharmacogenetic investigations, the results should be interpreted with caution. Although these results require replication, the data suggest preliminarily that the DRD2-Taq1A polymorphism may influence treatment response to bupropion for smoking cessation and, further, that exploration of gene x gene and gene x craving interactions in future, larger studies may provide mechanistic insights into the complex pharmacodynamics of bupropion.
Figures

References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994.
-
- Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Human Molecular Genetics. 1997;6:577–582. - PubMed
-
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, Sulser F. Bupropion: A review of its mechanism of antidepressant activity. Journal of Clinical Psychiatry. 1995;56:395–401. - PubMed
-
- Audrain J, Boyd NR, Roth J, Main D, Caporaso NF, Lerman C. Genetic susceptibility testing in smoking-cessation treatment: One-year outcomes of a randomized trial. Addictive Behaviors. 1997;22:741–751. - PubMed
-
- Baker TB, Hatsukami DK, Lerman C, O’Malley SS, Shields AE, Fiore MC. Transdisciplinary science applied to the evaluation of treatments for tobacco use. Nicotine & Tobacco Research. 2003;5(Suppl 1):S89–S99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

