Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models
- PMID: 17654495
- PMCID: PMC3065715
- DOI: 10.1002/jcp.21209
Tissue stretch decreases soluble TGF-beta1 and type-1 procollagen in mouse subcutaneous connective tissue: evidence from ex vivo and in vivo models
Abstract
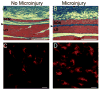
Transforming growth factor beta 1 (TGF-beta1) plays a key role in connective tissue remodeling, scarring, and fibrosis. The effects of mechanical forces on TGF-beta1 and collagen deposition are not well understood. We tested the hypothesis that brief (10 min) static tissue stretch attenuates TGF-beta1-mediated new collagen deposition in response to injury. We used two different models: (1) an ex vivo model in which excised mouse subcutaneous tissue (N = 44 animals) was kept in organ culture for 4 days and either stretched (20% strain for 10 min 1 day after excision) or not stretched; culture media was assayed by ELISA for TGF-beta1; (2) an in vivo model in which mice (N = 22 animals) underwent unilateral subcutaneous microsurgical injury on the back, then were randomized to stretch (20-30% strain for 10 min twice a day for 7 days) or no stretch; subcutaneous tissues of the back were immunohistochemically stained for Type-1 procollagen. In the ex vivo model, TGF-beta1 protein was lower in stretched versus non-stretched tissue (repeated measures ANOVA, P < 0.01). In the in vivo model, microinjury resulted in a significant increase in Type-1 procollagen in the absence of stretch (P < 0.001), but not in the presence of stretch (P = 0.21). Thus, brief tissue stretch attenuated the increase in both soluble TGF-beta1 (ex vivo) and Type-1 procollagen (in vivo) following tissue injury. These results have potential relevance to the mechanisms of treatments applying brief mechanical stretch to tissues (e.g., physical therapy, respiratory therapy, mechanical ventilation, massage, yoga, acupuncture).
(c) 2007 Wiley-Liss, Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

