Osmotic loading of spherical gels: a biomimetic study of hindered transport in the cell protoplasm
- PMID: 17655471
- PMCID: PMC2828939
- DOI: 10.1115/1.2746371
Osmotic loading of spherical gels: a biomimetic study of hindered transport in the cell protoplasm
Abstract
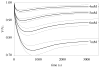
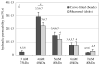
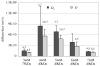
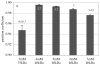
Osmotic loading of cells has been used to investigate their physicochemical properties as well as their biosynthetic activities. The classical Kedem-Katchalsky framework for analyzing cell response to osmotic loading, which models the cell as a fluid-filled membrane, does not generally account for the possibility of partial volume recovery in response to loading with a permeating osmolyte, as observed in some experiments. The cell may be more accurately represented as a hydrated gel surrounded by a semi-permeable membrane, with the gel and membrane potentially exhibiting different properties. To help assess whether this more elaborate model of the cell is justified, this study investigates the response of spherical gels to osmotic loading, both from experiments and theory. The spherical gel is described using the framework of mixture theory. In the experimental component of the study alginate is used as the model gel, and is osmotically loaded with dextran solutions of various concentrations and molecular weight, to verify the predictions from the theoretical analysis. Results show that the mixture framework can accurately predict the transient and equilibrium response of alginate gels to osmotic loading with dextran solutions. It is found that the partition coefficient of dextran in alginate regulates the equilibrium volume response and can explain partial volume recovery based on passive transport mechanisms. The validation of this theoretical framework facilitates future investigations of the role of the protoplasm in the response of cells to osmotic loading.
Figures




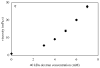
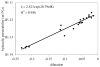
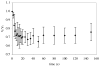
Similar articles
-
A mixture theory analysis for passive transport in osmotic loading of cells.J Biomech. 2006;39(3):464-75. doi: 10.1016/j.jbiomech.2004.12.013. J Biomech. 2006. PMID: 16389086 Free PMC article.
-
Ion exchange in alginate gels--dynamic behaviour revealed by electron paramagnetic resonance.Soft Matter. 2015 Dec 14;11(46):8968-74. doi: 10.1039/c5sm02062j. Epub 2015 Sep 24. Soft Matter. 2015. PMID: 26399427
-
Mechanical and diffusive properties of homogeneous alginate gels in form of particles and cylinders.J Biomed Mater Res A. 2008 Dec 1;87(3):808-18. doi: 10.1002/jbm.a.31680. J Biomed Mater Res A. 2008. PMID: 18228267
-
Enzyme-catalyzed phase transition of alginate gels and gelatin-alginate interpenetrated networks.Biomacromolecules. 2007 Nov;8(11):3613-8. doi: 10.1021/bm700767u. Epub 2007 Oct 19. Biomacromolecules. 2007. PMID: 17949036
-
Alginate gel particles-A review of production techniques and physical properties.Crit Rev Food Sci Nutr. 2017 Apr 13;57(6):1133-1152. doi: 10.1080/10408398.2014.965773. Crit Rev Food Sci Nutr. 2017. PMID: 25976619 Review.
Cited by
-
In silico Mechano-Chemical Model of Bone Healing for the Regeneration of Critical Defects: The Effect of BMP-2.PLoS One. 2015 Jun 4;10(6):e0127722. doi: 10.1371/journal.pone.0127722. eCollection 2015. PLoS One. 2015. PMID: 26043112 Free PMC article.
-
Finite Element Formulation of Multiphasic Shell Elements for Cell Mechanics Analyses in FEBio.J Biomech Eng. 2018 Aug 3;140(12):121009. doi: 10.1115/1.4041043. Online ahead of print. J Biomech Eng. 2018. PMID: 30098156 Free PMC article.
-
Novel nanofiber-based scaffold for rotator cuff repair and augmentation.Tissue Eng Part A. 2009 Jan;15(1):115-26. doi: 10.1089/ten.tea.2008.0014. Tissue Eng Part A. 2009. PMID: 18788982 Free PMC article.
-
A Hybrid Reactive Multiphasic Mixture With a Compressible Fluid Solvent.J Biomech Eng. 2022 Jan 1;144(1):011013. doi: 10.1115/1.4051926. J Biomech Eng. 2022. PMID: 34318318 Free PMC article.
-
Nonlinear osmotic properties of the cell nucleus.Ann Biomed Eng. 2009 Mar;37(3):477-91. doi: 10.1007/s10439-008-9618-5. Epub 2008 Dec 24. Ann Biomed Eng. 2009. PMID: 19107599 Free PMC article.
References
-
- Onsager L. Reciprocal Relations in Irreversible Processes. I. Phys Rev. 1931;37:405–426.
-
- Onsager L. Reciprocal Relations in Irreversible Processes. Ii. Phys Rev. 1931;38:2265–2279.
-
- Staverman AJ. Non-Equilibrium Thermodynamics of Membrane Processes. Tr Faraday Soc. 1952;48:176–185.
-
- Kedem O, Katchalsky A. Thermodynamic Analysis of the Permeability of Biological Membranes to Non-Electrolytes. Biochim Biophys Acta. 1958;27:229–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

