The Pseudomonas putida Lon protease is involved in N-acyl homoserine lactone quorum sensing regulation
- PMID: 17655747
- PMCID: PMC1949823
- DOI: 10.1186/1471-2180-7-71
The Pseudomonas putida Lon protease is involved in N-acyl homoserine lactone quorum sensing regulation
Abstract
Background: In Pseudomonas putida and Pseduomonas aeruginosa, the similar PpuR/RsaL/PpuI and LasR/RsaL/LasI acyl homoserine lactones (AHLs) quorum sensing (QS) systems have been shown to be under considerable regulation by other global regulators. A major regulator is the RsaL protein which strongly directly represses the transcription of the P. putida ppuI and P. aeruginosa lasI AHL synthases. In this study we screened a transposon mutant bank of P. putida in order to identify if any other regulators were involved in negative regulation of AHL QS.
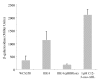
Results: In our screen we identified three Tn5 mutants which displayed overproduction of AHLs in P. putida strain WCS358. Two of the mutants had a Tn5 located in the rsaL gene, whereas in one mutant the transposon was located in the lon protease gene. Lon proteases play important roles in protein quality control via degradation of misfolded proteins. It was determined that in the P. putida lon mutant, AHL levels, PpuR levels and ppuI promoter activity all increased significantly; we therefore postulated that PpuR is a target for Lon. The Lon protease had no effect on AHL production in P. aeruginosa.
Conclusion: The Lon protease is a negative regulator of AHL production in P. putida WCS358. The Lon protease has also been shown by others to influence AHL QS in Vibrio fischeri and Agrobacterium tumefaciens and can thus become an important regulator of AHL QS timing and regulation in bacteria.
Figures





Similar articles
-
Regulation of the N-acyl homoserine lactone-dependent quorum-sensing system in rhizosphere Pseudomonas putida WCS358 and cross-talk with the stationary-phase RpoS sigma factor and the global regulator GacA.Appl Environ Microbiol. 2004 Sep;70(9):5493-502. doi: 10.1128/AEM.70.9.5493-5502.2004. Appl Environ Microbiol. 2004. PMID: 15345437 Free PMC article.
-
The ppuI-rsaL-ppuR quorum-sensing system regulates biofilm formation of Pseudomonas putida PCL1445 by controlling biosynthesis of the cyclic lipopeptides putisolvins I and II.J Bacteriol. 2006 Apr;188(8):2898-906. doi: 10.1128/JB.188.8.2898-2906.2006. J Bacteriol. 2006. PMID: 16585751 Free PMC article.
-
Functional characterization of the quorum sensing regulator RsaL in the plant-beneficial strain Pseudomonas putida WCS358.Appl Environ Microbiol. 2012 Feb;78(3):726-34. doi: 10.1128/AEM.06442-11. Epub 2011 Nov 23. Appl Environ Microbiol. 2012. PMID: 22113916 Free PMC article.
-
An evolving perspective on the Pseudomonas aeruginosa orphan quorum sensing regulator QscR.Front Cell Infect Microbiol. 2014 Oct 28;4:152. doi: 10.3389/fcimb.2014.00152. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25389523 Free PMC article. Review.
-
Impact of quorum sensing on fitness of Pseudomonas aeruginosa.Int J Med Microbiol. 2006 Apr;296(2-3):93-102. doi: 10.1016/j.ijmm.2006.01.043. Epub 2006 Feb 28. Int J Med Microbiol. 2006. PMID: 16503417 Review.
Cited by
-
Dissection of the cis-2-decenoic acid signaling network in Pseudomonas aeruginosa using microarray technique.Front Microbiol. 2015 Apr 28;6:383. doi: 10.3389/fmicb.2015.00383. eCollection 2015. Front Microbiol. 2015. PMID: 25972860 Free PMC article.
-
Maintenance of the virulence plasmid in Shigella flexneri is influenced by Lon and two functional partitioning systems.Mol Microbiol. 2019 May;111(5):1355-1366. doi: 10.1111/mmi.14225. Epub 2019 Mar 22. Mol Microbiol. 2019. PMID: 30767313 Free PMC article.
-
The Two-Component System CpxRA Negatively Regulates the Locus of Enterocyte Effacement of Enterohemorrhagic Escherichia coli Involving σ(32) and Lon protease.Front Cell Infect Microbiol. 2016 Feb 5;6:11. doi: 10.3389/fcimb.2016.00011. eCollection 2016. Front Cell Infect Microbiol. 2016. Retraction in: Front Cell Infect Microbiol. 2025 Jun 12;15:1639671. doi: 10.3389/fcimb.2025.1639671. PMID: 26904510 Free PMC article. Retracted.
-
Negative regulation of quorum-sensing systems in Pseudomonas aeruginosa by ATP-dependent Lon protease.J Bacteriol. 2008 Jun;190(12):4181-8. doi: 10.1128/JB.01873-07. Epub 2008 Apr 11. J Bacteriol. 2008. PMID: 18408026 Free PMC article.
-
Essential roles of Lon protease in the morpho-physiological traits of the rice pathogen Burkholderia glumae.PLoS One. 2021 Sep 15;16(9):e0257257. doi: 10.1371/journal.pone.0257257. eCollection 2021. PLoS One. 2021. PMID: 34525127 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

