Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis
- PMID: 17655751
- PMCID: PMC1963326
- DOI: 10.1186/1750-1172-2-34
Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis
Abstract
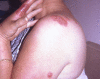
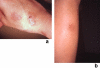
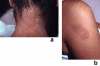
Sweet's syndrome (the eponym for acute febrile neutrophilic dermatosis) is characterized by a constellation of clinical symptoms, physical features, and pathologic findings which include fever, neutrophilia, tender erythematous skin lesions (papules, nodules, and plaques), and a diffuse infiltrate consisting predominantly of mature neutrophils that are typically located in the upper dermis. Several hundreds cases of Sweet's syndrome have been published. Sweet's syndrome presents in three clinical settings: classical (or idiopathic), malignancy-associated, and drug-induced. Classical Sweet's syndrome (CSS) usually presents in women between the age of 30 to 50 years, it is often preceded by an upper respiratory tract infection and may be associated with inflammatory bowel disease and pregnancy. Approximately one-third of patients with CSS experience recurrence of the dermatosis. The malignancy-associated Sweet's syndrome (MASS) can occur as a paraneoplastic syndrome in patients with an established cancer or individuals whose Sweet's syndrome-related hematologic dyscrasia or solid tumor was previously undiscovered; MASS is most commonly related to acute myelogenous leukemia. The dermatosis can precede, follow, or appear concurrent with the diagnosis of the patient's cancer. Hence, MASS can be the cutaneous harbinger of either an undiagnosed visceral malignancy in a previously cancer-free individual or an unsuspected cancer recurrence in an oncology patient. Drug-induced Sweet's syndrome (DISS) most commonly occurs in patients who have been treated with granulocyte-colony stimulating factor, however, other medications may also be associated with DISS. The pathogenesis of Sweet's syndrome may be multifactorial and still remains to be definitively established. Clinical and laboratory evidence suggests that cytokines have an etiologic role. Systemic corticosteroids are the therapeutic gold standard for Sweet's syndrome. After initiation of treatment with systemic corticosteroids, there is a prompt response consisting of dramatic improvement of both the dermatosis-related symptoms and skin lesions. Topical application of high potency corticosteroids or intralesional corticosteroids may be efficacious for treating localized lesions. Other first-line oral systemic agents are potassium iodide and colchicine. Second-line oral systemic agents include indomethacin, clofazimine, cyclosporine, and dapsone. The symptoms and lesions of Sweet's syndrome may resolved spontaneously, without any therapeutic intervention; however, recurrence may follow either spontaneous remission or therapy-induced clinical resolution.
Figures







References
-
- Cohen PR. Sweet's syndrome. Orphanet Encyclopedia. 2003. http://www.orpha.net/data/patho/GB/uk-Sweet.pdf (accessed 25 July 2007).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

