Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis
- PMID: 17656432
- PMCID: PMC2277191
- DOI: 10.1113/jphysiol.2007.137034
Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis
Abstract
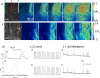
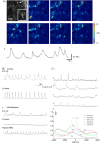
Electrical rhythmicity in the renal pelvis provides the fundamental drive for the peristaltic contractions that propel urine from the kidney to bladder for storage until micturition. Although atypical smooth muscles (ASMCs) within the most proximal regions of the renal pelvis have long been implicated as the pacemaker cells, the presence of a sparsely distributed population of rhythmically active Kit-positive interstitial cells of Cajal-like cells (ICC-LCs) have confounded our understanding of pelviureteric peristalsis. We have recorded the electrical activity and separately visualized changes in intracellular Ca(2+) concentration in typical smooth muscle cells (TSMCs), ASMCs and ICC-LCs using intracellular microelectrodes and a fluorescent Ca(2+) indicator, fluo-4. Nifedipine (1-10 microm)-sensitive driven action potentials and Ca(2+) waves (frequency 6-15 min(-1)) propagated through the TSMC layer at a velocity of 1-2 mm s(-1). High frequency (10-40 min(-1)) Ca(2+) transients and spontaneous transient depolarizations (STDs) were recorded in ASMCs in the absence or presence of 1 microm nifedipine. ICC-LCs displayed low frequency (1-3 min(-1)) Ca(2+) transients which we speculated arose from cells that displayed action potentials with long plateaus (2-5 s). Neither electrical activity propagated over distances > 50 microm. In 1 microm nifedipine, ASMCs or ICC-LCs separated by < 30 microm displayed some synchronicity in their Ca(2+) transient discharge suggesting that they may well be acting as 'point sources' of excitation to the TSMC layer. We speculate that ASMCs act as the primary pacemaker in the renal pelvis while ICC-LCs play a supportive role, but can take over pacemaking in the absence of the proximal pacemaker drive.
Figures









References
-
- Constantinou CE, Neubarth JL, Mensah-Dwumah M. Frequency gradient in the autorhythmicity of the pyeloureteral pacemaker system. Experientia. 1978;34:614–615. - PubMed
-
- David SG, Cebrian C, Vaughan ED, Herzlinger D. C-kit and ureteral peristalsis. J Urol. 2005;173:292–295. - PubMed
-
- Dixon JS, Gosling JA. The fine structure of pacemaker cells in the pig renal calices. Anat Rec. 1973;175:139–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

