N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration
- PMID: 17656467
- PMCID: PMC2597693
- DOI: 10.1096/fj.07-8396com
N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration
Abstract
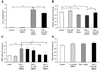
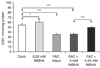
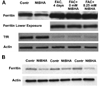
Age-related macular degeneration (AMD) is the leading cause of severe visual impairment in the elderly in developed countries. AMD patients have elevated levels of iron within the retinal pigment epithelia (RPE), which may lead to oxidative damage to mitochondria, disruption of retinal metabolism, and vision impairment or loss. As a possible model for iron-induced AMD, we investigated the effects of excess iron in cultured human fetal RPE cells on oxidant levels and mitochondrial cytochrome c oxidase (complex IV) function and tested for protection by N-tert-butyl hydroxylamine (NtBHA), a known mitochondrial antioxidant. RPE exposure to ferric ammonium citrate resulted in a time- and dose-dependent increase in intracellular iron, which increased oxidant production and decreased glutathione (GSH) levels and mitochondrial complex IV activity. NtBHA addition to iron-overloaded RPE cells led to a reduction of intracellular iron content, oxidative stress, and partial restoration of complex IV activity and GSH content. NtBHA might be useful in AMD due to its potential to reduce oxidative stress, mitochondrial damage, and age-related iron accumulation, which may damage normal RPE function and lead to loss of vision.
Figures



Similar articles
-
Iron induced oxidative damage as a potential factor in age-related macular degeneration: the Cogan Lecture.Invest Ophthalmol Vis Sci. 2006 Nov;47(11):4660-4. doi: 10.1167/iovs.06-0568. Invest Ophthalmol Vis Sci. 2006. PMID: 17065470
-
Establishing chronic models of age-related macular degeneration via long-term iron ion overload.Am J Physiol Cell Physiol. 2024 May 1;326(5):C1367-C1383. doi: 10.1152/ajpcell.00532.2023. Epub 2024 Feb 26. Am J Physiol Cell Physiol. 2024. PMID: 38406826
-
Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD.Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2728-36. doi: 10.1167/iovs.07-1472. Epub 2008 Mar 7. Invest Ophthalmol Vis Sci. 2008. PMID: 18326691 Free PMC article.
-
Iron and age-related macular degeneration.Klin Oczna. 2009;111(4-6):174-7. Klin Oczna. 2009. PMID: 19673453 Review.
-
Oxidative damage and protection of the RPE.Prog Retin Eye Res. 2000 Mar;19(2):205-21. doi: 10.1016/s1350-9462(99)00009-9. Prog Retin Eye Res. 2000. PMID: 10674708 Review.
Cited by
-
Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy.Mol Neurodegener. 2021 Oct 18;16(1):71. doi: 10.1186/s13024-021-00479-8. Mol Neurodegener. 2021. PMID: 34663413 Free PMC article. Review.
-
Role of iron in ischemia-induced neurodegeneration: mechanisms and insights.Metab Brain Dis. 2014 Sep;29(3):583-91. doi: 10.1007/s11011-014-9522-7. Epub 2014 Mar 11. Metab Brain Dis. 2014. PMID: 24615430 Review.
-
Mitochondrial dysfunction in retinal diseases.Curr Eye Res. 2011 Dec;36(12):1069-77. doi: 10.3109/02713683.2011.607536. Epub 2011 Oct 6. Curr Eye Res. 2011. PMID: 21978133 Free PMC article. Review.
-
Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging.Antioxid Redox Signal. 2010 Apr;12(4):503-35. doi: 10.1089/ars.2009.2598. Antioxid Redox Signal. 2010. PMID: 19650712 Free PMC article. Review.
-
Prevention of Oxidative Stress-Induced Retinal Pigment Epithelial Cell Death by the PPARgamma Agonists, 15-Deoxy-Delta 12, 14-Prostaglandin J(2).PPAR Res. 2008;2008:720163. doi: 10.1155/2008/720163. PPAR Res. 2008. PMID: 18382621 Free PMC article.
References
-
- Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology. 2003;49:1–11. - PubMed
-
- Hahn P, Ying GS, Beard J, Dunaief JL. Iron levels in human retina: sex difference and increase with age. NeuroReport. 2006;17:1803–1806. - PubMed
-
- Hahn P, Milam AH, Dunaief JL. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch’s membrane. Arch. Ophthalmol. 2003;121:1099–1105. - PubMed
-
- Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic. Biol. Med. 1997;23:783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

