Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone
- PMID: 17656470
- PMCID: PMC2216427
- DOI: 10.1681/ASN.2007030348
Thiazide diuretics directly induce osteoblast differentiation and mineralized nodule formation by interacting with a sodium chloride co-transporter in bone
Abstract
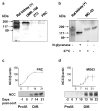
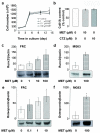
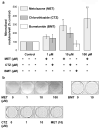
Thiazide diuretics are used worldwide as a first-choice drug for patients with uncomplicated hypertension. In addition to their antihypertensive effect, thiazides increase bone mineral density and reduce the prevalence of fractures. Traditionally, these effects have been attributed to increased renal calcium reabsorption that occurs secondary to the inhibition of the thiazide-sensitive sodium chloride cotransporter (NCC) in the distal tubule. The aim of the current study was to determine whether thiazides exert a direct bone-forming effect independent of their renal action. We found that the osteoblasts of human and rat bone also express NCC, suggesting that these bone-forming cells may be an additional target for thiazides. In vitro, NCC protein was virtually absent in proliferating human and fetal rat osteoblasts, whereas its expression dramatically increased during differentiation. Thiazides did not affect osteoblast proliferation, but directly stimulated the production of the osteoblast differentiation markers runt-related transcription factor 2 (runx2) and osteopontin. Using overexpression/knockdown studies in fetal rat calvarial cells, we show that thiazides increase the formation of mineralized nodules, but loop diuretics do not. Overall, our study demonstrates that thiazides directly stimulate osteoblast differentiation and bone mineral formation independent of their effects in the kidney. Therefore, in addition to their use as antihypertensive drugs, our results suggest that thiazides may find a role in the prevention and treatment of osteoporosis.
Figures


References
-
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85(2):423–493. - PubMed
-
- Wasnich RD, Benfante RJ, Yano K, Heilbrun L, Vogel JM. Thiazide effect on the mineral content of bone. N Engl J Med. 1983;309(6):344–347. - PubMed
-
- LaCroix AZ, Wienpahl J, White LR, Wallace RB, Scherr PA, George LK, Cornoni-Huntley J, Ostfeld AM. Thiazide diuretic agents and the incidence of hip fracture. N Engl J Med. 1990;322(5):286–290. - PubMed
-
- Jones G, Nguyen T, Sambrook PN, Eisman JA. Thiazide diuretics and fractures: can meta-analysis help? J Bone Miner Res. 1995;10(1):106–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

