Reactivation of methionine synthase from Thermotoga maritima (TM0268) requires the downstream gene product TM0269
- PMID: 17656578
- PMCID: PMC2203375
- DOI: 10.1110/ps.072936307
Reactivation of methionine synthase from Thermotoga maritima (TM0268) requires the downstream gene product TM0269
Abstract
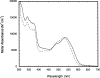
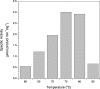
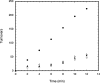
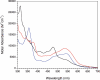
The crystal structure of the Thermotoga maritima gene product TM0269, determined as part of genome-wide structural coverage of T. maritima by the Joint Center for Structural Genomics, revealed structural homology with the fourth module of the cobalamin-dependent methionine synthase (MetH) from Escherichia coli, despite the lack of significant sequence homology. The gene specifying TM0269 lies in close proximity to another gene, TM0268, which shows sequence homology with the first three modules of E. coli MetH. The fourth module of E. coli MetH is required for reductive remethylation of the cob(II)alamin form of the cofactor and binds the methyl donor for this reactivation, S-adenosylmethionine (AdoMet). Measurements of the rates of methionine formation in the presence and absence of TM0269 and AdoMet demonstrate that both TM0269 and AdoMet are required for reactivation of the inactive cob(II)alamin form of TM0268. These activity measurements confirm the structure-based assignment of the function of the TM0269 gene product. In the presence of TM0269, AdoMet, and reductants, the measured activity of T. maritima MetH is maximal near 80 degrees C, where the specific activity of the purified protein is approximately 15% of that of E. coli methionine synthase (MetH) at 37 degrees C. Comparisons of the structures and sequences of TM0269 and the reactivation domain of E. coli MetH suggest that AdoMet may be bound somewhat differently by the homologous proteins. However, the conformation of a hairpin that is critical for cobalamin binding in E. coli MetH, which constitutes an essential structural element, is retained in the T. maritima reactivation protein despite striking divergence of the sequences.
Figures


References
-
- Bandarian V. and Matthews, R.G. 2001. Quantitation of rate enhancements attained by the binding of cobalamin to methionine synthase. Biochemistry 40: 5056–5064. - PubMed
-
- Bandarian V., Pattridge, K.A., Lennon, B.W., Huddler, D.P., Matthews, R.G., and Ludwig, M.L. 2002. Domain alternation switches B12-dependent methionine synthase to the activation conformation. Nat. Struct. Biol. 9: 53–56. - PubMed
-
- Banerjee R.V., Johnston, N.L., Sobeski, J.K., Datta, P., and Matthews, R.G. 1989. Cloning and sequence analysis of the Escherichia coli metH gene encoding cobalamin-dependent methionine synthase and isolation of a tryptic fragment containing the cobalamin-binding domain. J. Biol. Chem. 264: 13888–13895. - PubMed
-
- Banerjee R.V., Harder, S.F., Ragsdale, S.W., and Matthews, R.G. 1990. Mechanism of reductive activation of cobalamin-dependent methionine synthase: An electron paramagnetic resonance spectroelectrochemical study. Biochemistry 29: 1129–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

