Physical-chemical determinants of turn conformations in globular proteins
- PMID: 17656584
- PMCID: PMC2203374
- DOI: 10.1110/ps.072898507
Physical-chemical determinants of turn conformations in globular proteins
Abstract
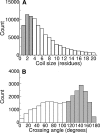
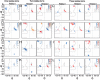
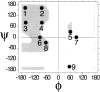
Globular proteins are assemblies of alpha-helices and beta-strands, interconnected by reverse turns and longer loops. Most short turns can be classified readily into a limited repertoire of discrete backbone conformations, but the physical-chemical determinants of these distinct conformational basins remain an open question. We investigated this question by exhaustive analysis of all backbone conformations accessible to short chain segments bracketed by either an alpha-helix or a beta-strand (i.e., alpha-segment-alpha, beta-segment-beta, alpha-segment-beta, and beta-segment-alpha) in a nine-state model. We find that each of these four secondary structure environments imposes its own unique steric and hydrogen-bonding constraints on the intervening segment, resulting in a limited repertoire of conformations. In greater detail, an exhaustive set of conformations was generated for short backbone segments having reverse-turn chain topology and bracketed between elements of secondary structure. This set was filtered, and only clash-free, hydrogen-bond-satisfied conformers having reverse-turn topology were retained. The filtered set includes authentic turn conformations, observed in proteins of known structure, but little else. In particular, over 99% of the alternative conformations failed to satisfy at least one criterion and were excluded from the filtered set. Furthermore, almost all of the remaining alternative conformations have close tolerances that would be too tight to accommodate side chains longer than a single beta-carbon. These results provide a molecular explanation for the observation that reverse turns between elements of regular secondary can be classified into a small number of discrete conformations.
Figures


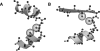
References
-
- Baldwin R.L. 2003. In search of the energetic role of peptide hydrogen bonds. J. Biol. Chem. 278: 17581–17588. - PubMed
-
- Brunet A.P., Huang, E.S., Huffine, M.E., Loeb, J.E., Weltman, R.J., and Hecht, M.H. 1993. The role of turns in the structure of an α-helical protein. Nature 364: 355–358. - PubMed
-
- Chothia C., Levitt, M., and Richardson, D. 1981. Helix to helix packing in proteins. J. Mol. Biol. 145: 215–250. - PubMed
-
- Chou P.Y. and Fasman, G.D. 1977. β-Turns in proteins. J. Mol. Biol. 115: 135–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

